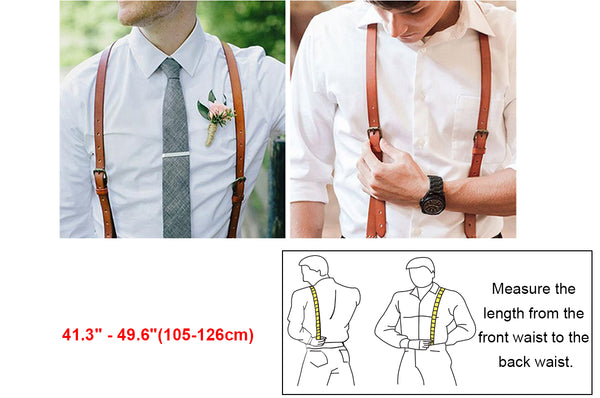
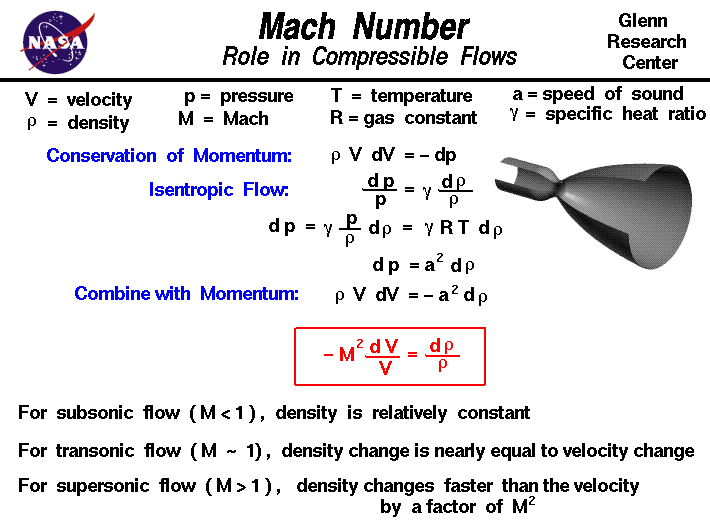
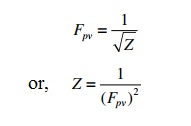2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1

Click here:point_up_2:to get an answer to your question :writing_hand:2 112153 1215 jals 42 5the compressibility factor for nitrogen at 330 k and 800
Click here👆to get an answer to your question ✍️ -2- 1-12-15 -3- 12-15- Jals -4- 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1-90 and 200 atm is 1-10-A certain mass of Noccupies a volume of 1 dmat 330 Kand eoo atm calculate volume occupied by same cuany of gas 750 K and 200 atm- -1- 1 L -2- 2L -3- 3L

Compression Factor Exam Problem using Molar Volumes - Fully
Answer in Civil and Environmental Engineering for emem #297959

Solved TABLE 1. Compressibility data for nitrogen N2 We also

Combined and Ideal Gas Law

Solved 2) Two liters of N2 at 0°C and 5 atm pressure are

2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor

At total pressure P_1 atm and P_2 atm N_2O_4 is dissociated to an
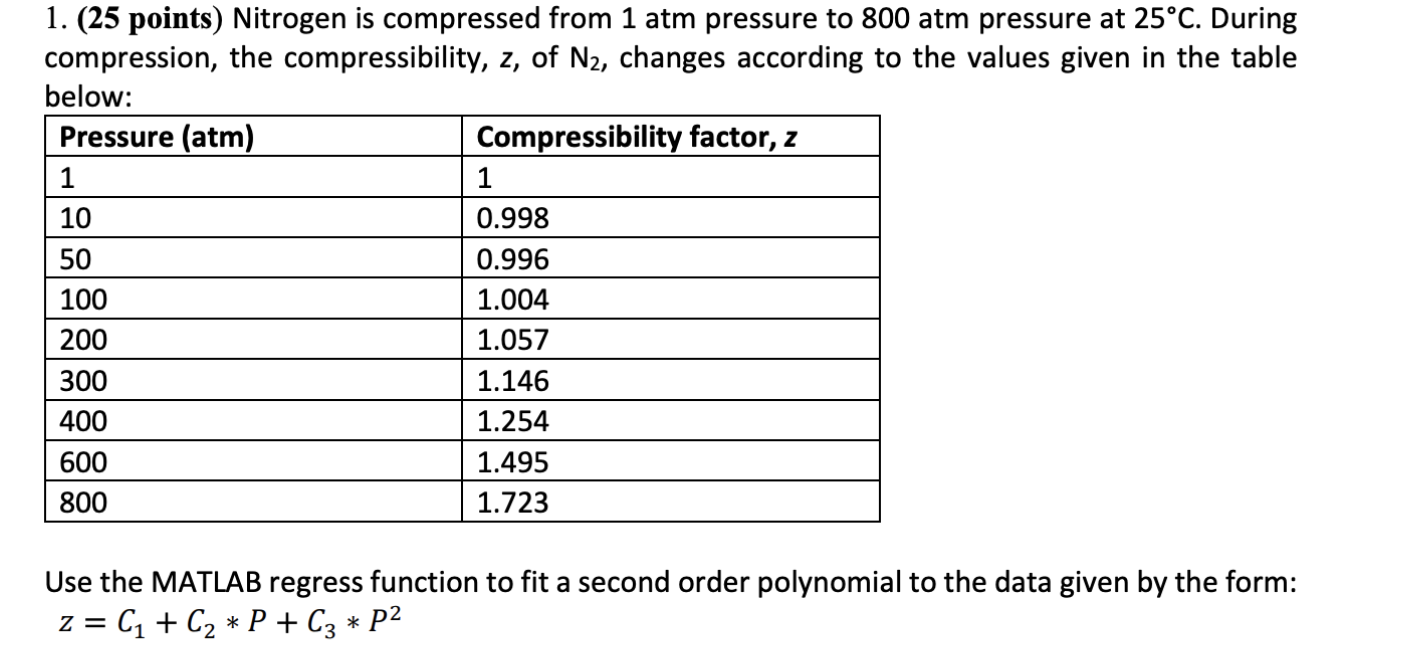
Solved 1. (25 points) Nitrogen is compressed from 1 atm
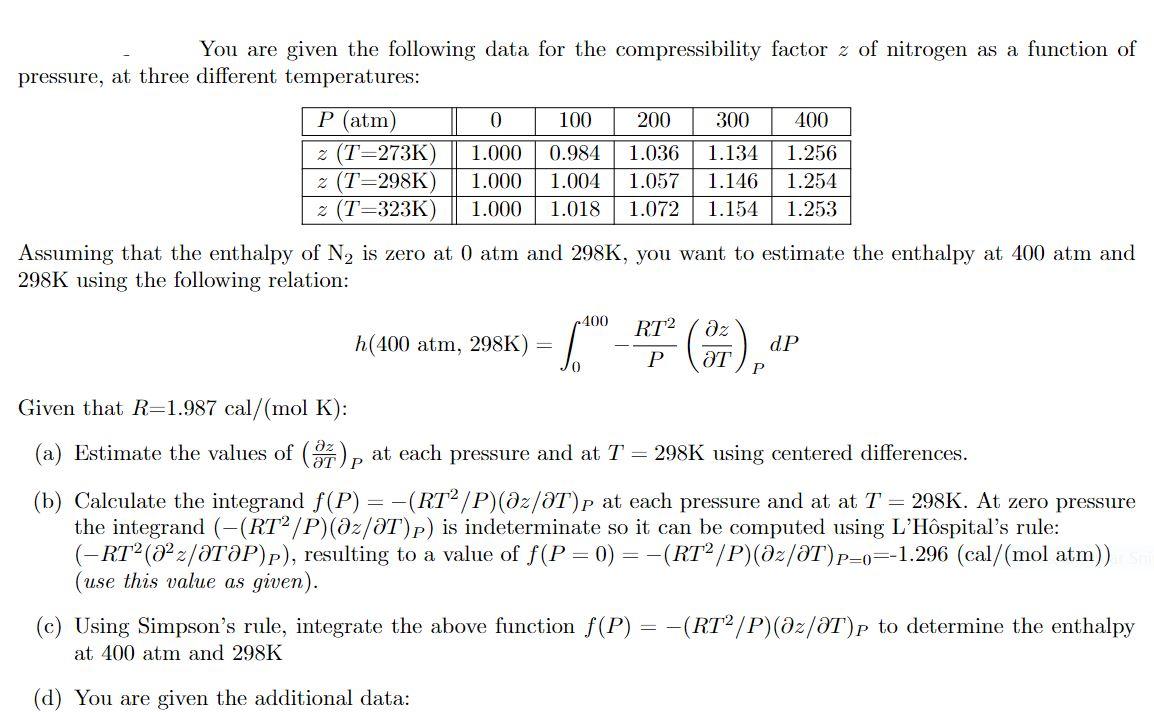
Solved You are given the following data for the

Solved Compressibility Chart 5.00 1.20 1.15 1.10 1.05 1.00

Solved 3.36) Determine the compressibility factor for

The compressibility factor for nitrogen at 330K and 800 atm is 1.90 an

The compressibility factor for nitrogen at 330K and 800 atm is 1.90 an