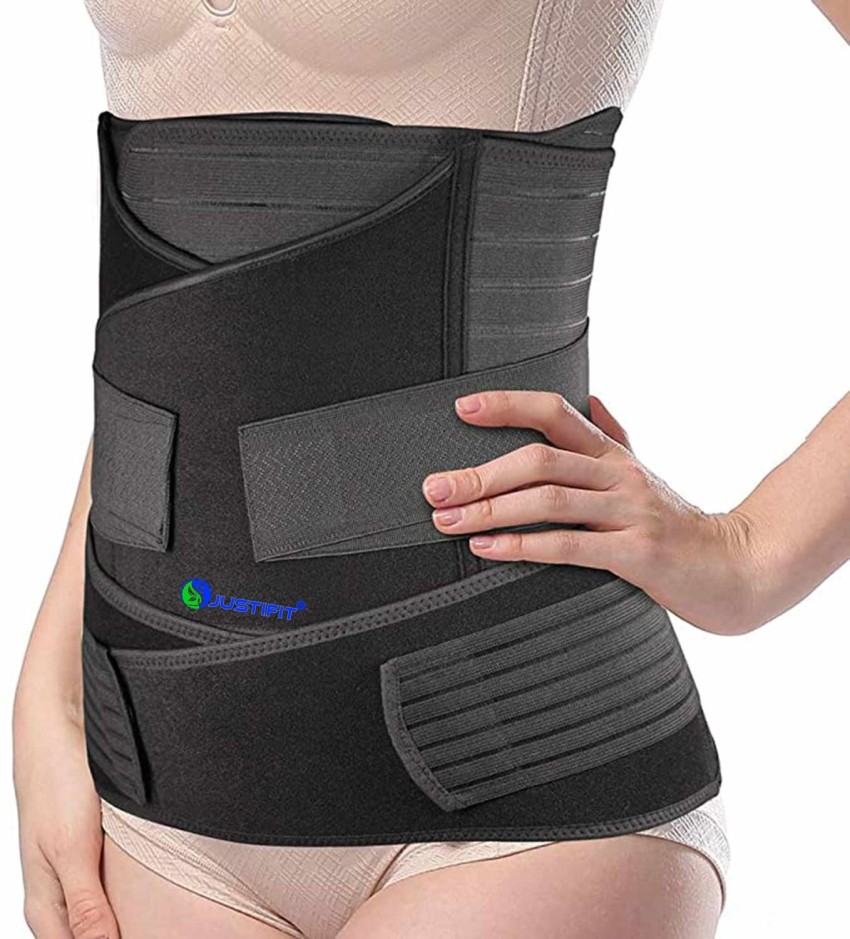
Solved FeBr3⟶Br2 F. F. G. 4. Which of the following is an
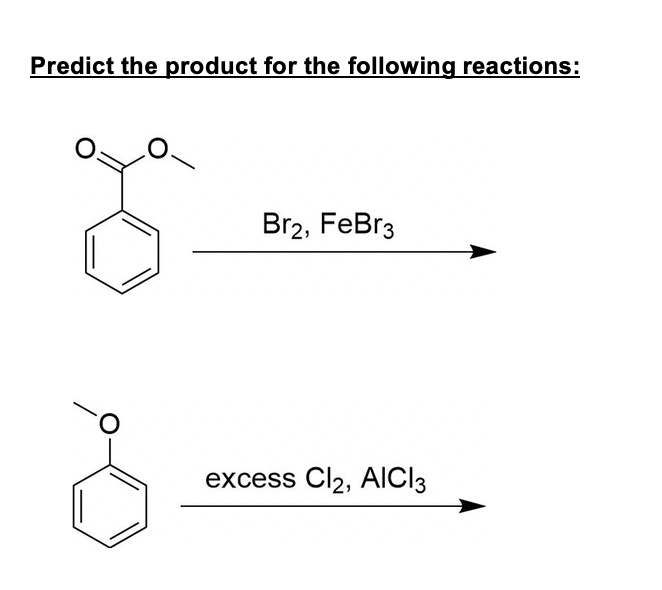
SOLVED: Predict the product for the following reactions: Br2 + FeBr3 excess Cl2 + AlCl3

How to Balance Fe + Br2 = FeBr3 (Iron + Bromine gas)

Solved 36 (1 Point) CO3+ + Ce 3+ Consider the following

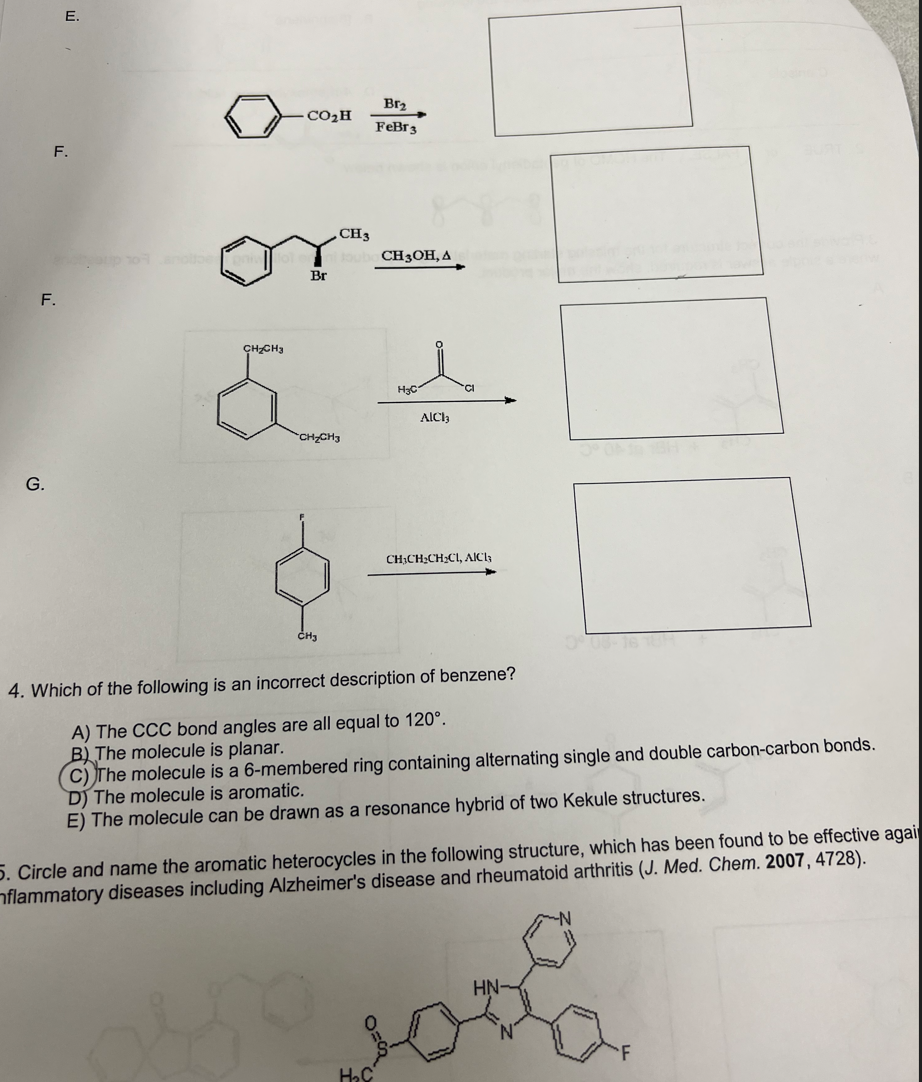
Organic Chemistry: Chapter 18 - Aromatic Substitution Reactions

How to Balance Fe + Br2 = FeBr3 (Iron + Bromine gas)
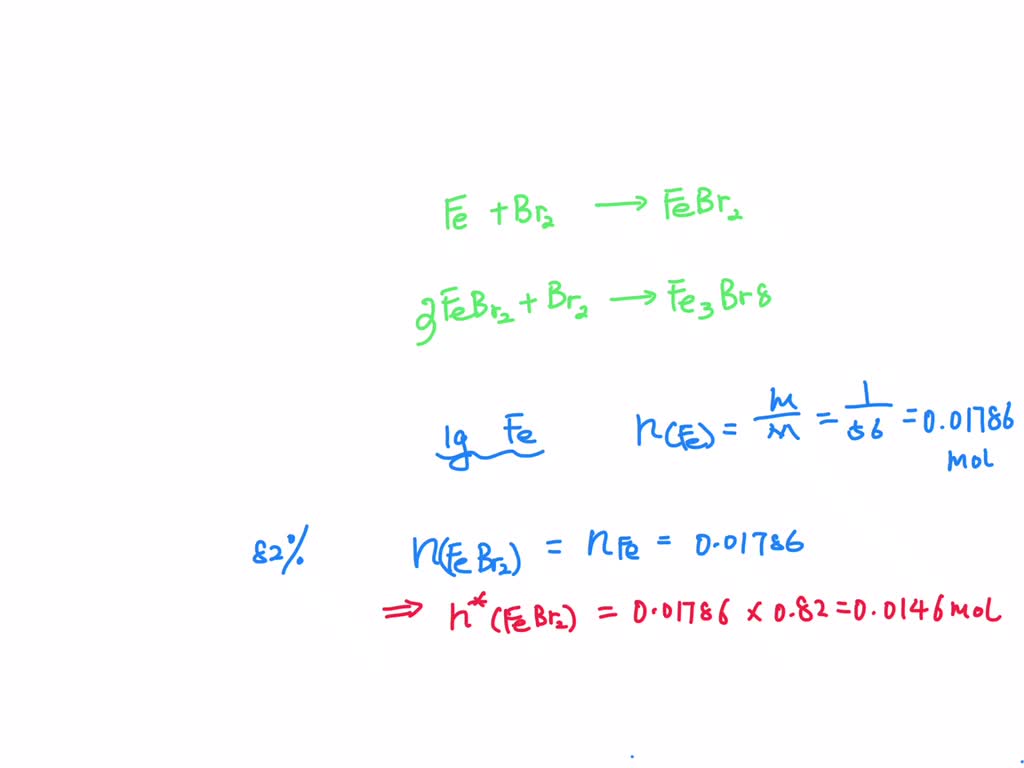
SOLVED: Given the following reactions: Fe + Br2 —-> FeBr2 3 FeBr2 + Br2 —> Fe3Br8 If the yield of each of the reactions is 82%, the mass in grams of Fe3Br8

Solved Identify the type of reaction expected for each of
The major product obtained on monobromination (with br2/ febr3)-Turito

Provide the product. Reagent: Br2, FeBr3

How to Balance Fe + Br2 = FeBr3 (Iron + Bromine gas)
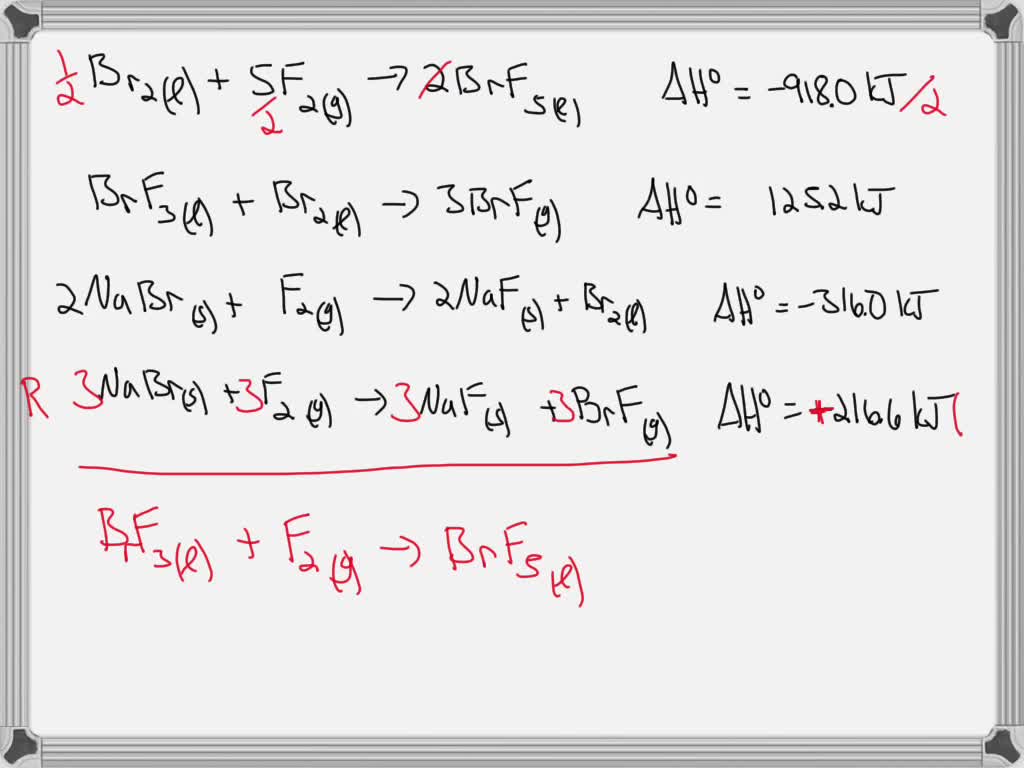
SOLVED: Given the following data: Br2(l) + 5F2(g) â†' 2BrF5(l) ΔH° = -918.0 kJ BrF3(l) + Br2(l) â†' 3BrF(g) ΔH° = 125.2 kJ 2NaBr(s) + F2(g) â†' 2NaF(s) + Br2(l) ΔH° =
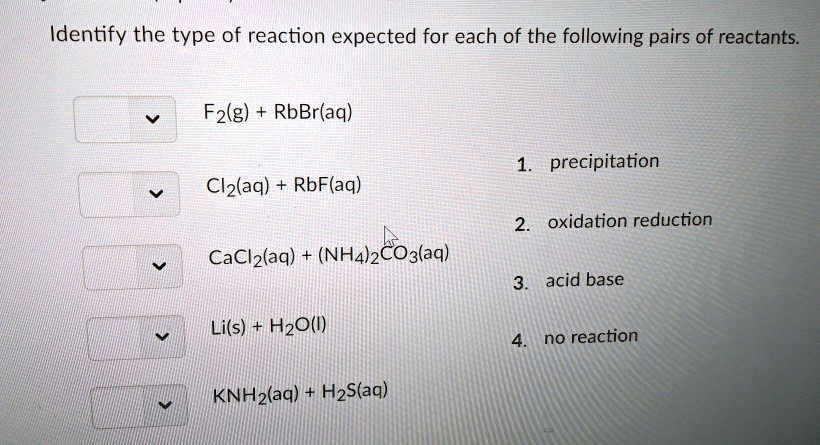
SOLVED: Identify the type of reaction expected for each of the
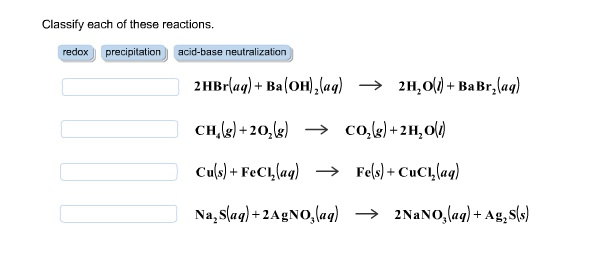
Solved Classify each of these reactions. 2HBr(aq) +

Complete the following reaction. + Br2, FeBr3, heat