An ideal gas is taken from (Pi , Vi ) to (Pi , Vi ) in three different ways. - Sarthaks eConnect
An ideal gas is taken from (Pi , Vi ) to (Pi , Vi ) in three different ways. Identify the process in (d) Equal work is done in Process A, B & C
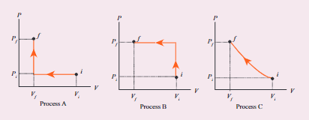
An ideal gas is taken fromPiVitoPfVfin three different ways Identify the process in which the work done on the gas the most

An ideal gas is taken from state A to state B via three different processes as shown in the pressure volume (P-V) diagram. If Q4, Q, & Q, indicates the heat absorbed

An ideal gas goes from state A to state B via three different processes as indicated in the P V diagram. If Q 1, Q 2, Q 3 indicate the heat absorbed
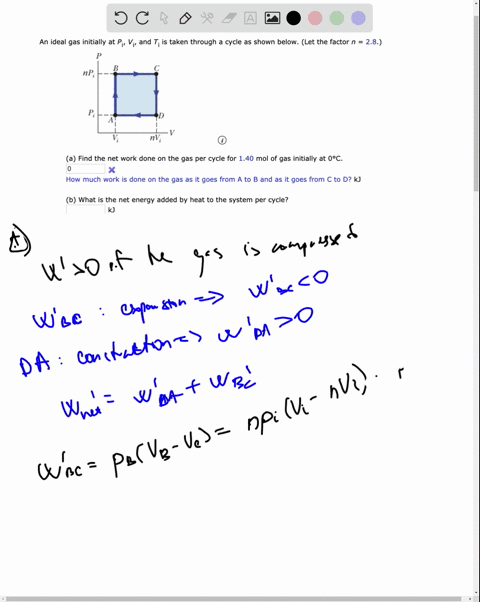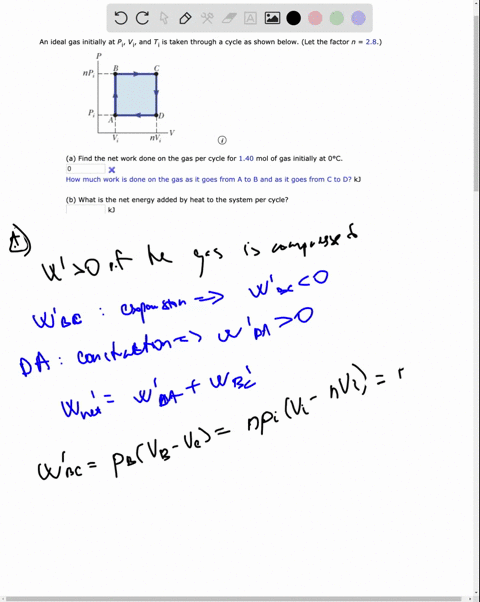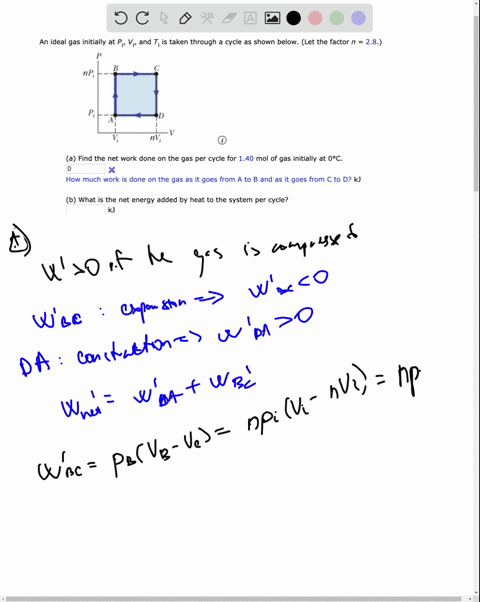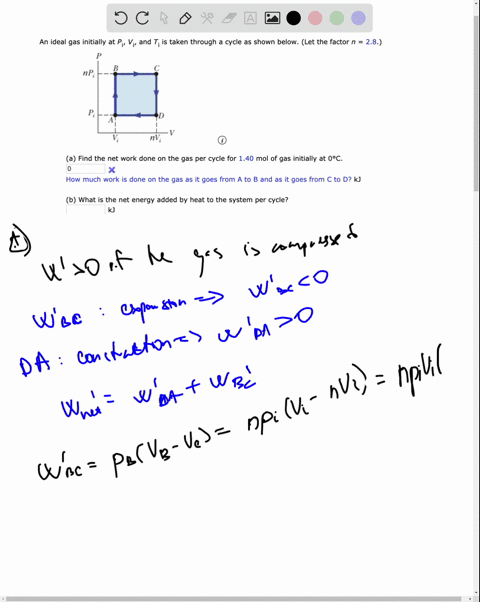
SOLVED: ideal gas initially at Pi, Vi, and Ti is taken through cycle as shown below: (Let the factor n 3.7.) nf Find the net work done on the gas per cycle

32 The change of the state of an ideal gas is presented by the diagram What is
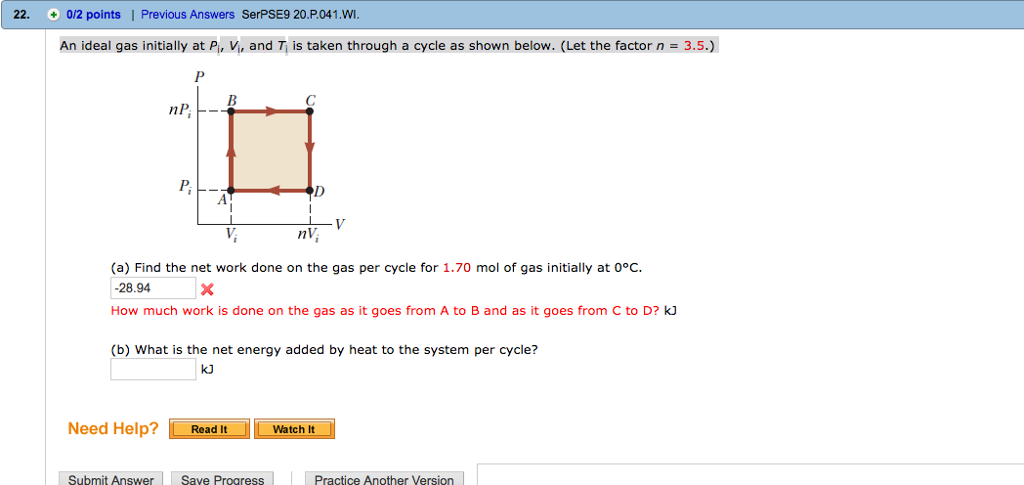
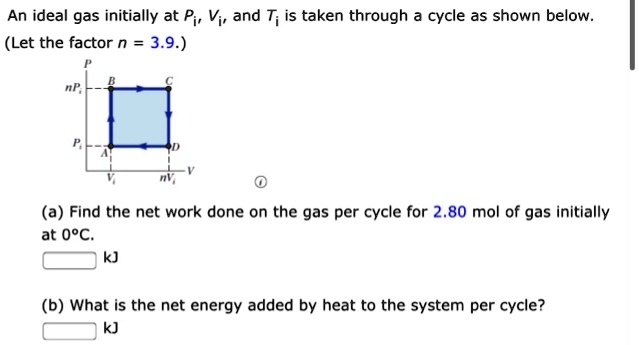
Solved An ideal gas initially at Pi, Vi, and Ti is taken

etwork cone zus ? / 3x1 Fix Skaws (P-V) diagram of an ideal gas under goes chance from A -> B, in the different process (1) In which work done is Maximum

Solved A system of ideal gas can be taken from state 3 to

etwork cone zus ? / 3x1 Fix Skaws (P-V) diagram of an ideal gas under goes chance from A -> B, in the different process (1) In which work done is Maximum
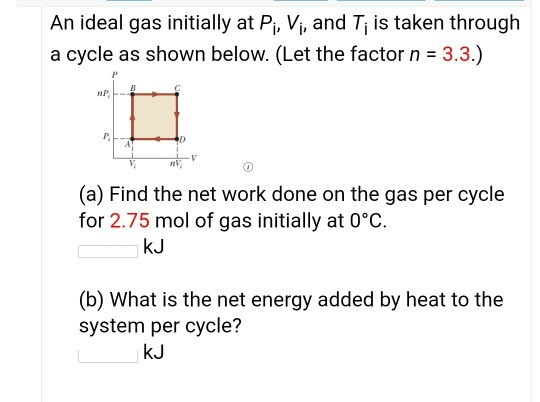
Solved An ideal gas initially at Pi, Vi, and Ti is taken
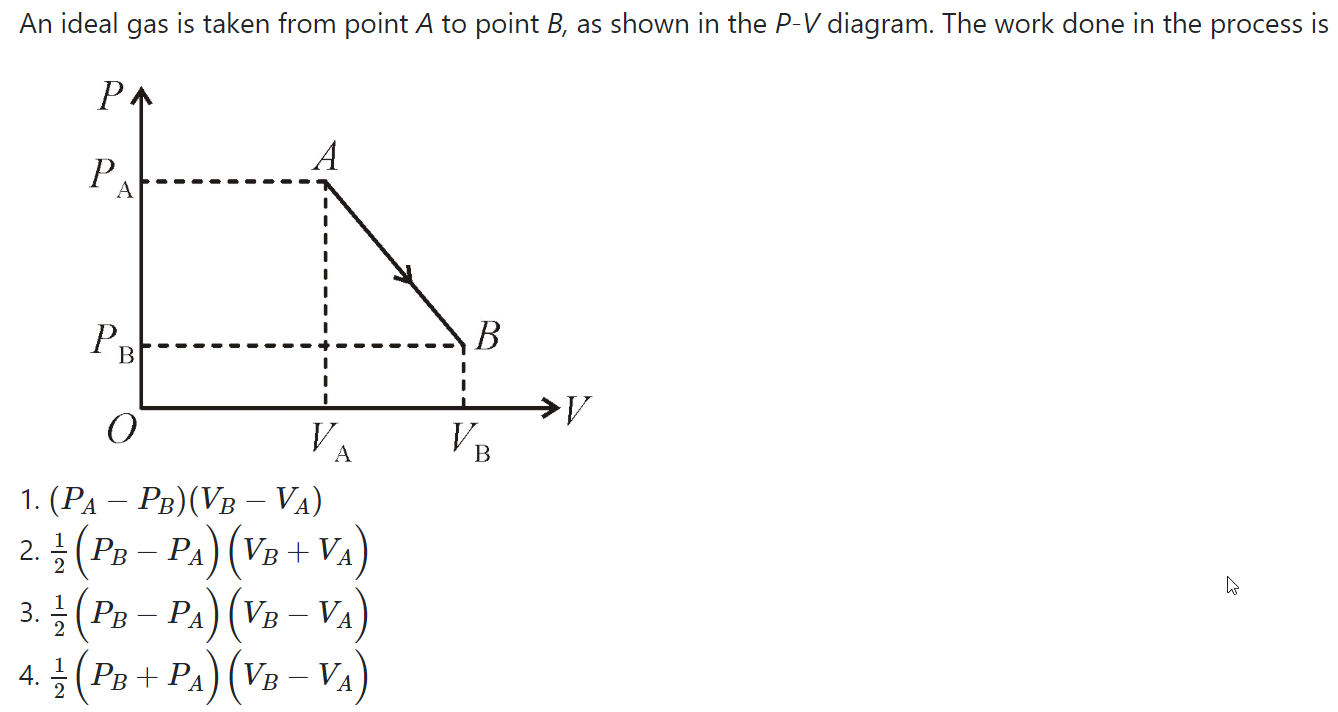
SOLVED: An ideal gas is taken from point A to point B, as shown in the P-V diagram. The work done in the process is 1. (PA-PB)(VB-VA) 2. (1)/(2)(PB-PA)(VB+VA) 3. (1)/(2)(PB-PA)(VB-VA) 4. (

Solved An ideal gas initially at Pi, Vi, and T is taken

An ideal gas is taken through the cycle `AtoBtoCtoA,` as shown in the figure, If the net heat