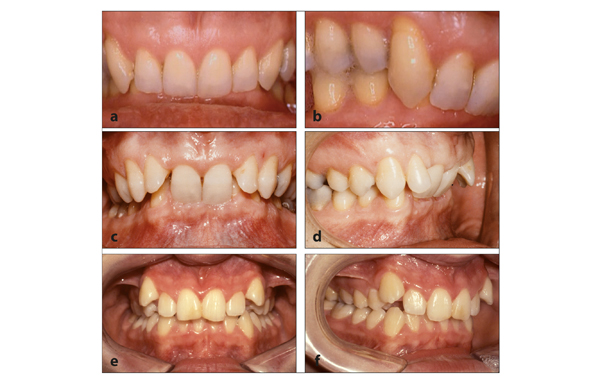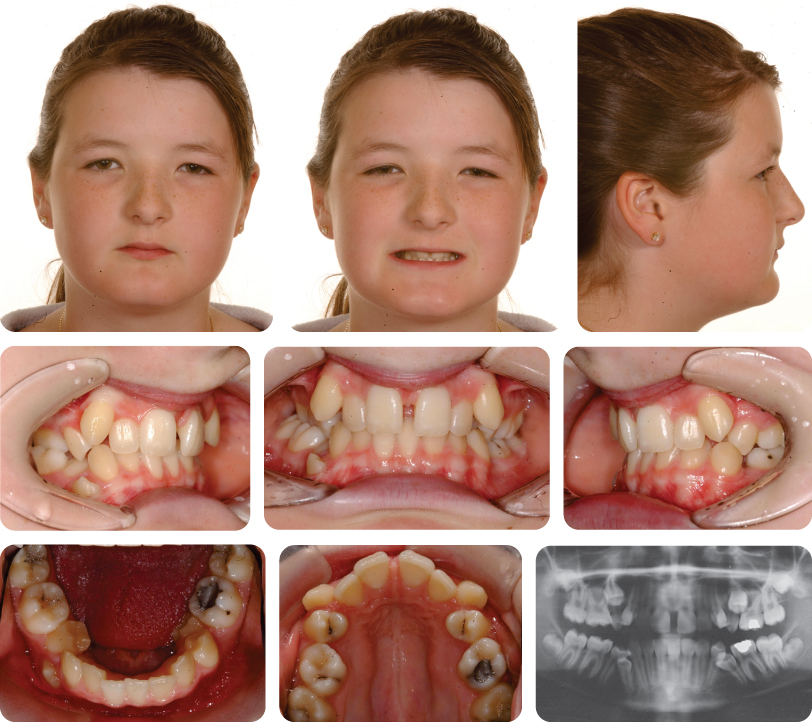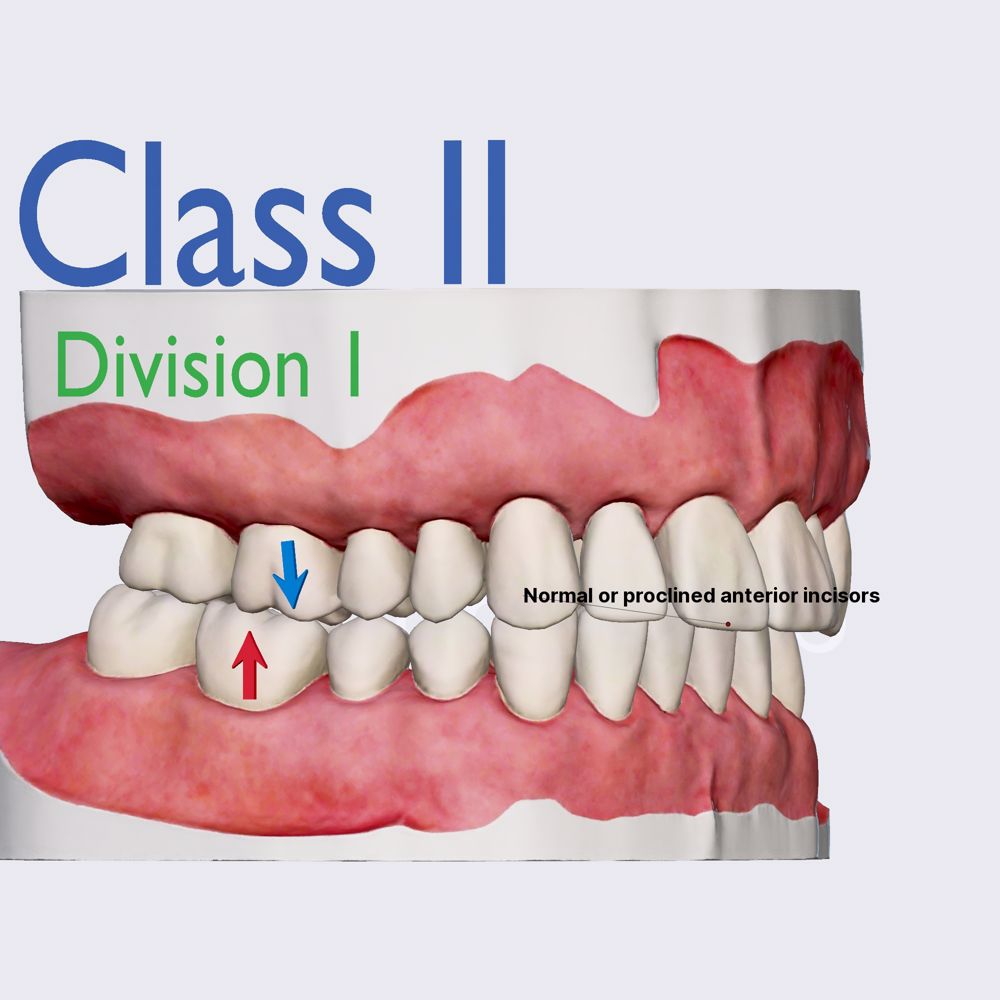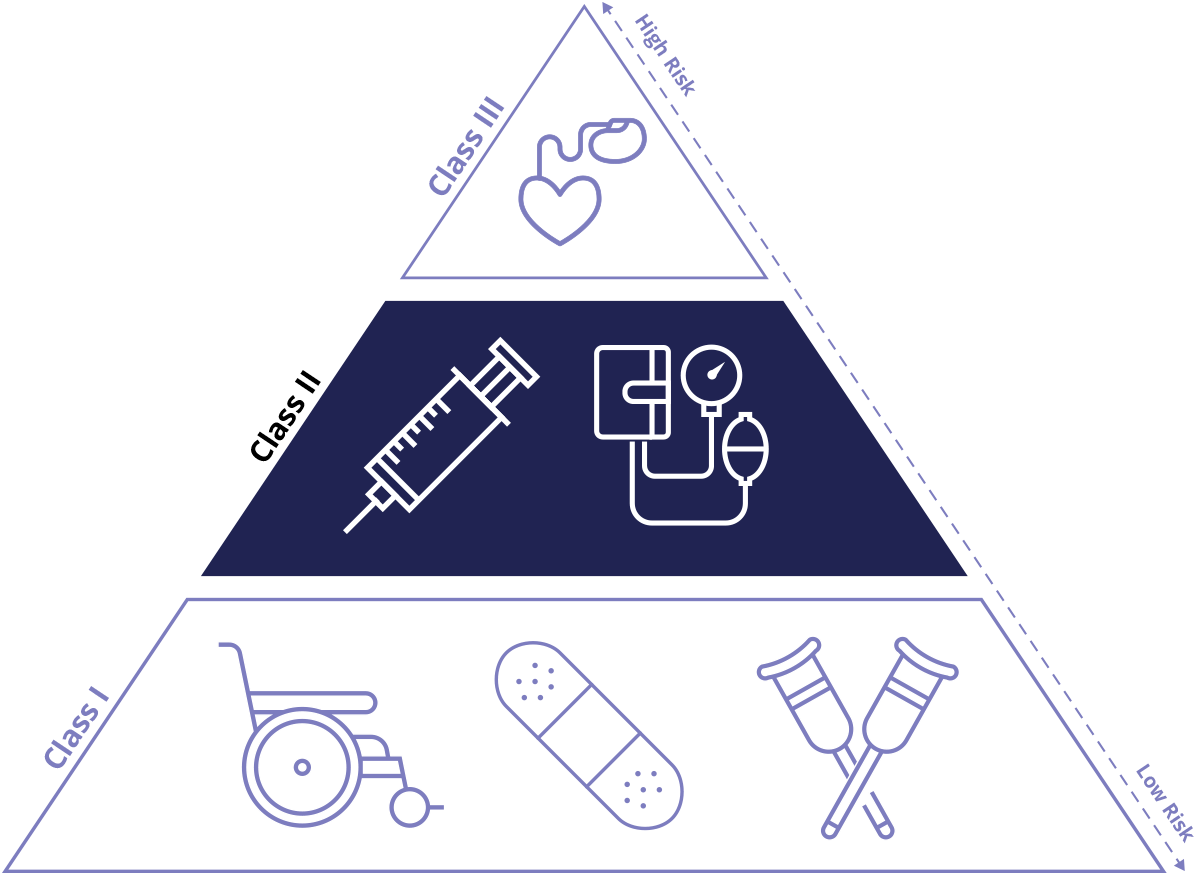
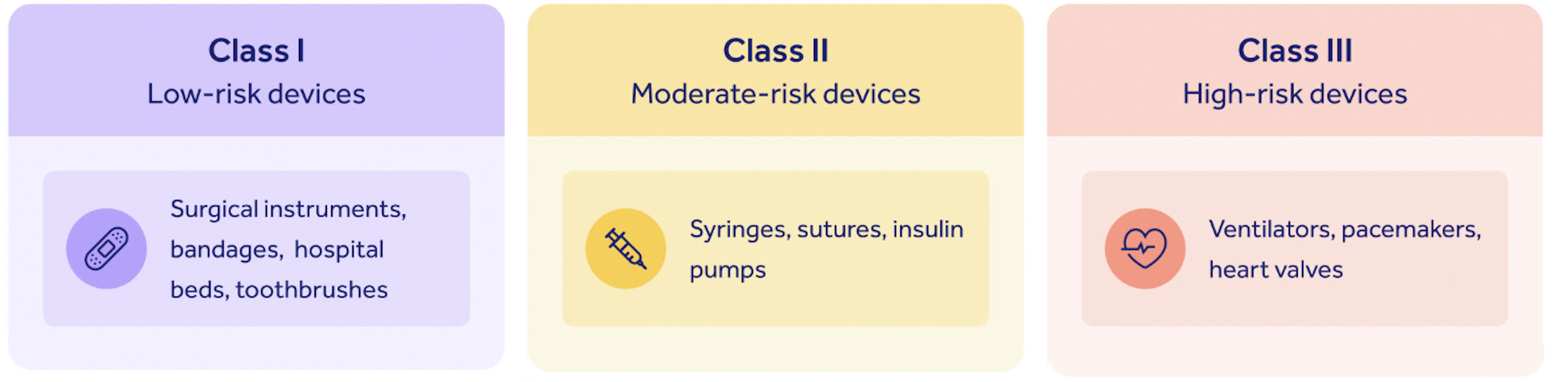
Class II medical devices have moderate to higher risks to patients or users. Over 40% of medical devices fall into this device category. The majority of medical devices are considered to be Class II devices. Some examples of Class II devices include catheters, syringes, contact lens, and pregnancy test kits.
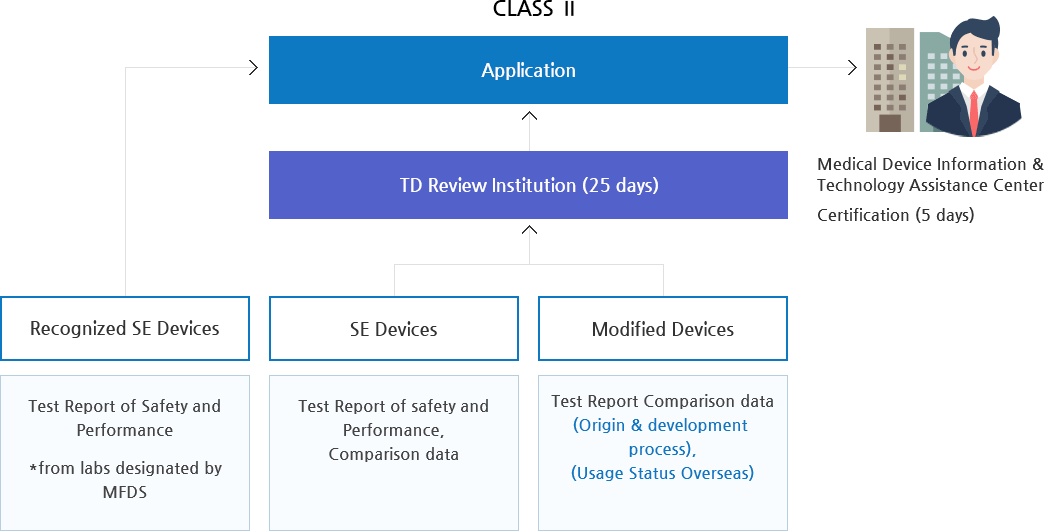
식품의약품안전처

All Class 1 Medical Device Manufacturers Must Meet These Specific EU MDR Requirements – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
510(k) exempt medical devices: how to tell if you need to submit
The 3 FDA medical device classes: differences and examples explained

Understanding FDA Device Classes Infographic
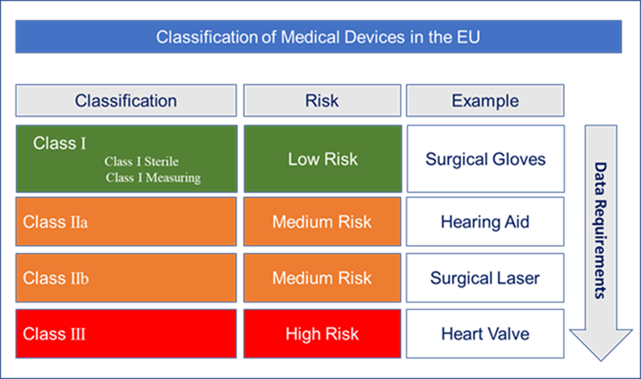
Approaching MDR Compliance

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

Classification of Medical device.

FDA Class II medical devices

A primer on medical device classification
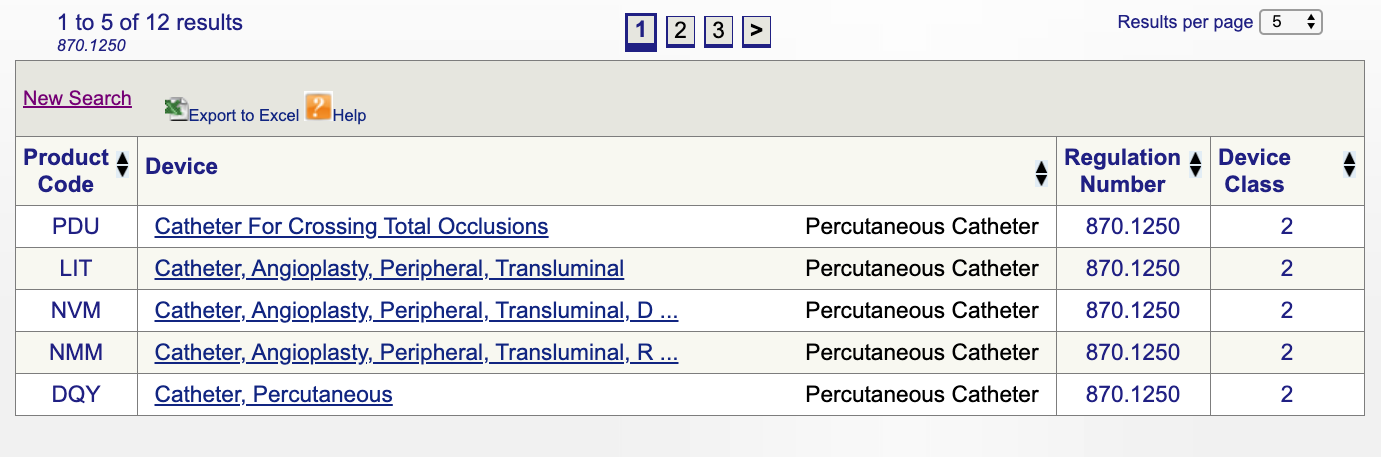
Medical Device Classification Guide - How To Determine Your Device Class