Cancer Biomarker Detection With Luminex Assays

Luminex and Bio-Techne are working together to support the development of early diagnostic solutions that detect cancer earlier through the use of proteomics.
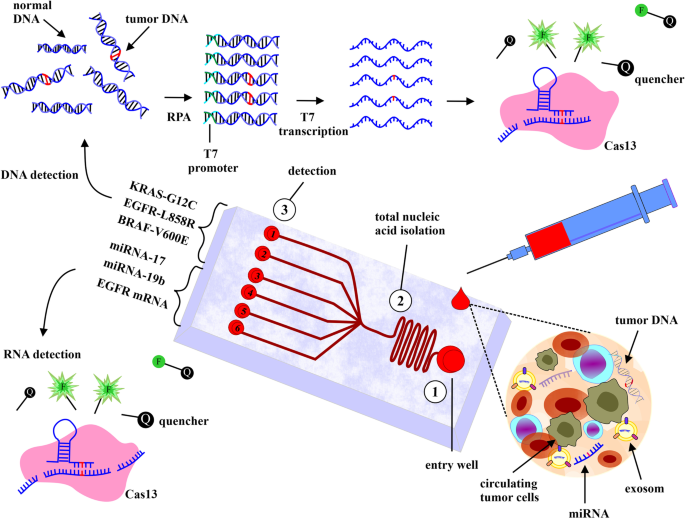
Charles Rosser, a research scientist and professor of biomedical sciences, has developed a multiplex test for early-stage bladder cancer using Luminex® xMAP® Technology. Bladder cancer affects over 570,000 people globally each year, and the Oncuria® test aims to detect bladder cancer, monitors for recurrence, and predicts which patients will benefit from the immunotherapy treatment. Current diagnostic assays lack sensitivity and do not provide a comprehensive view of the cancer’s molecular profile, but the Oncuria® test covers 10 glycoproteins to detect the biological signature of bladder cancer with sensitivity of 90% to 93% and specificity of 86% to 95%. The test received Breakthrough Device Designation status from the FDA and is currently available as a laboratory-developed test (LDT). In a recent clinical validation study involving about 350 patients, the test offered 93% sensitivity. Learn more about this major shift in the care of patients with bladder cancer.
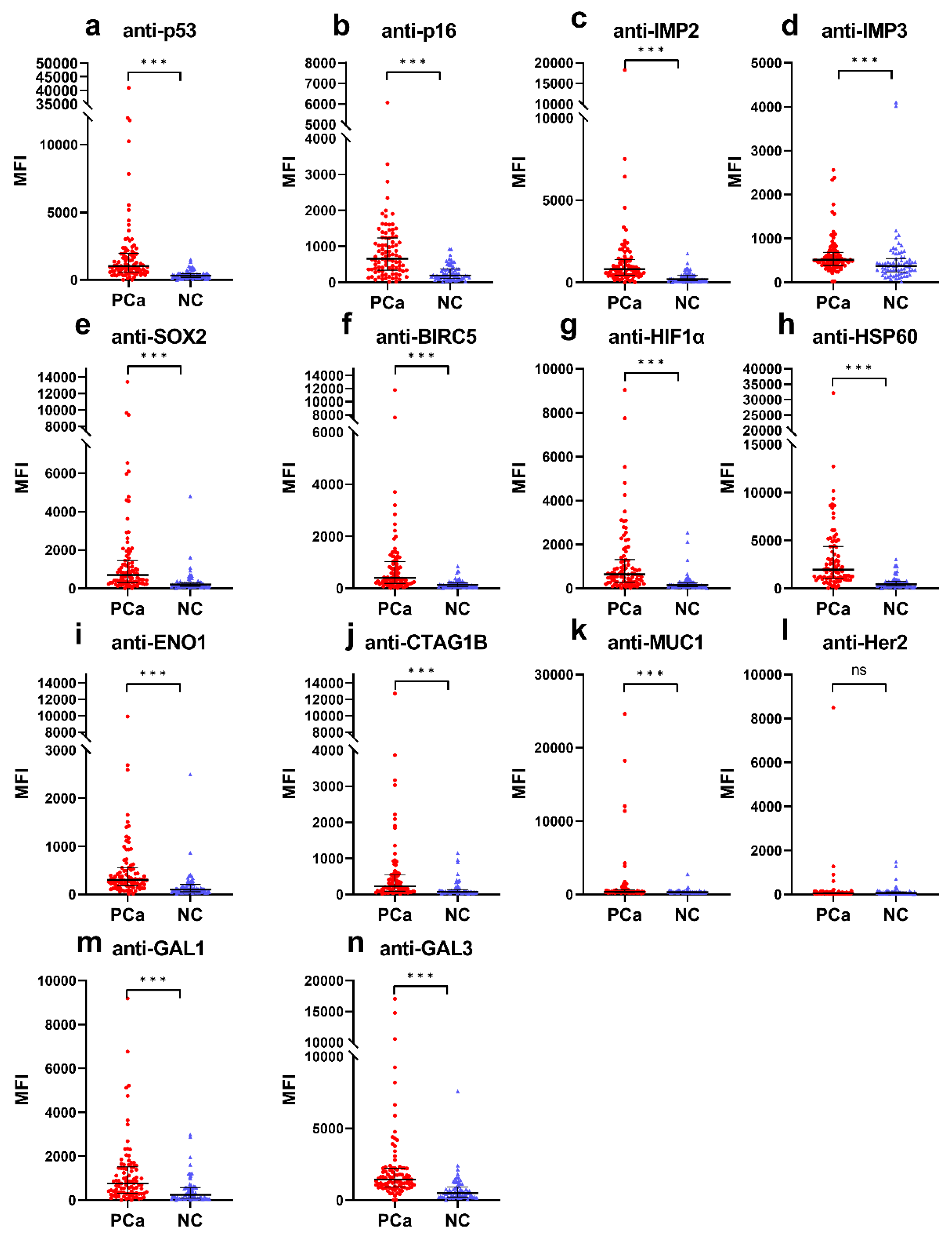
Cancers, Free Full-Text

Multiplexed Profiling of Extracellular Vesicles for Biomarker Development
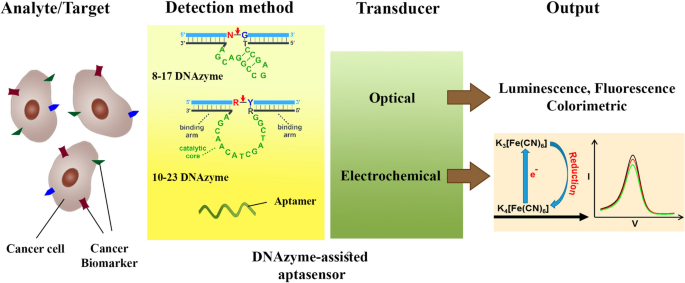
The recent advancements in the early detection of cancer biomarkers by DNAzyme-assisted aptasensors, Journal of Nanobiotechnology

Custom Luminex Assays: R&D Systems

Multiplex Cancer Assays for Cancer Biomarker Detection
Cross-Reactivity of Bio-Plex Pro™ Human Cancer Biomarker Assays to Mouse Proteins
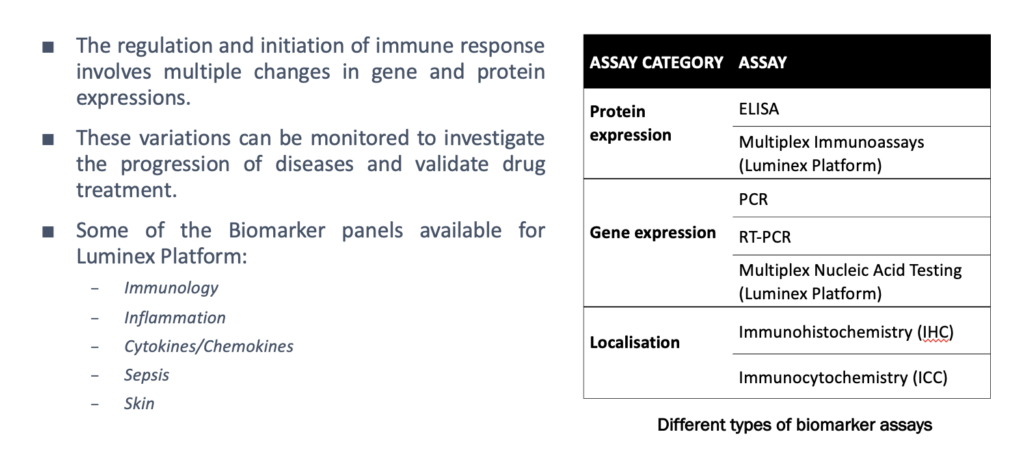
Immunoassays - Cellomatics Biosciences
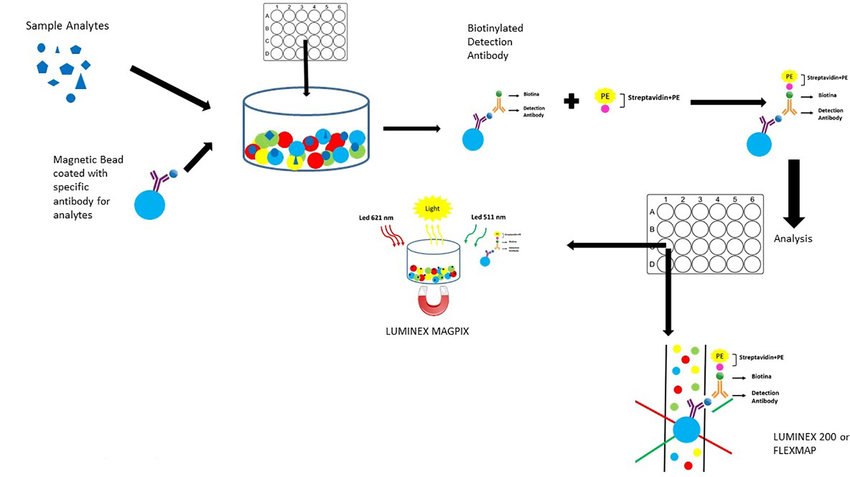
Application of Luminex Technology in Clinical and Scientific Research - Creative Proteomics
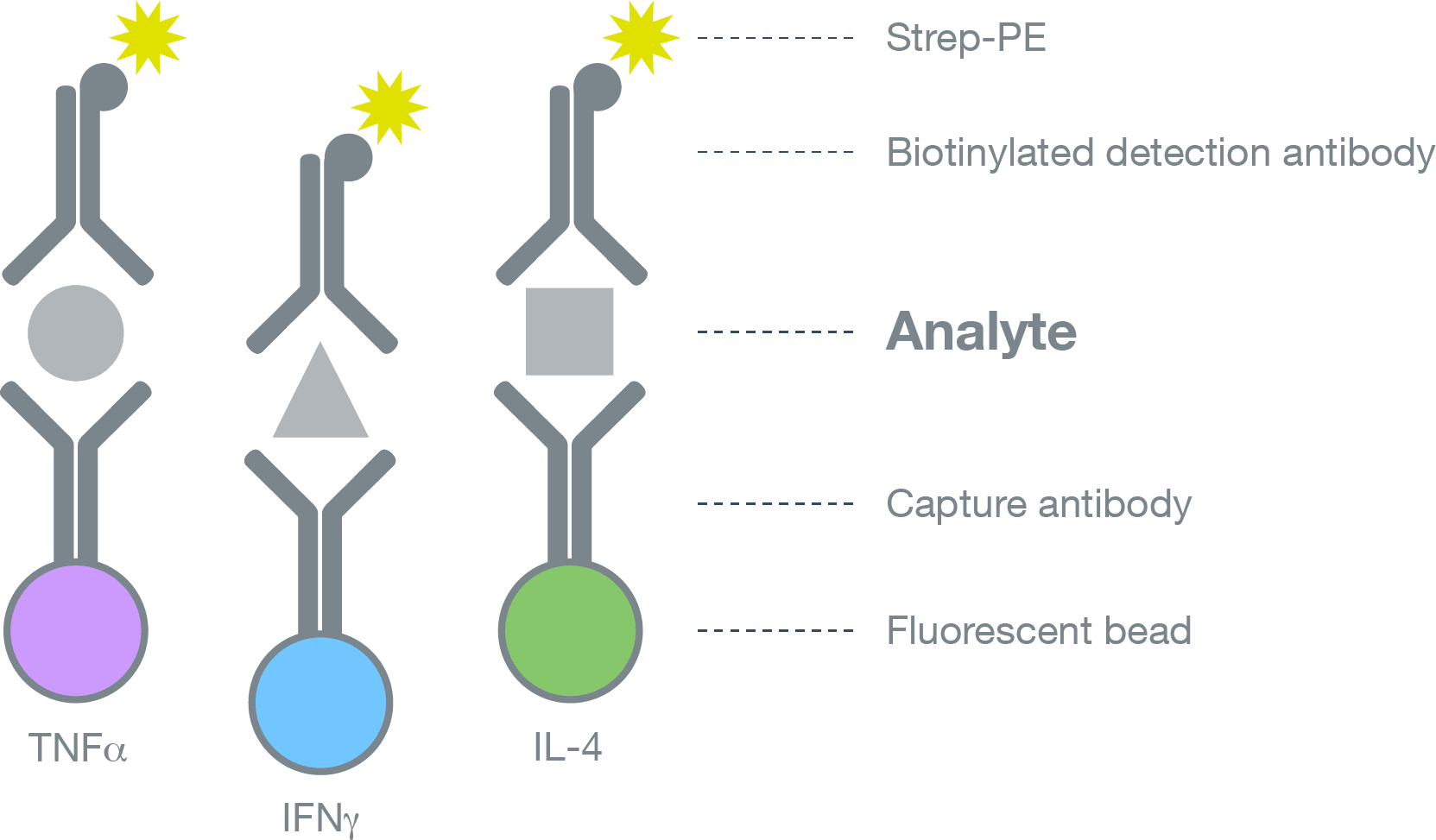
Multiplex Cytokine Assays with Luminex