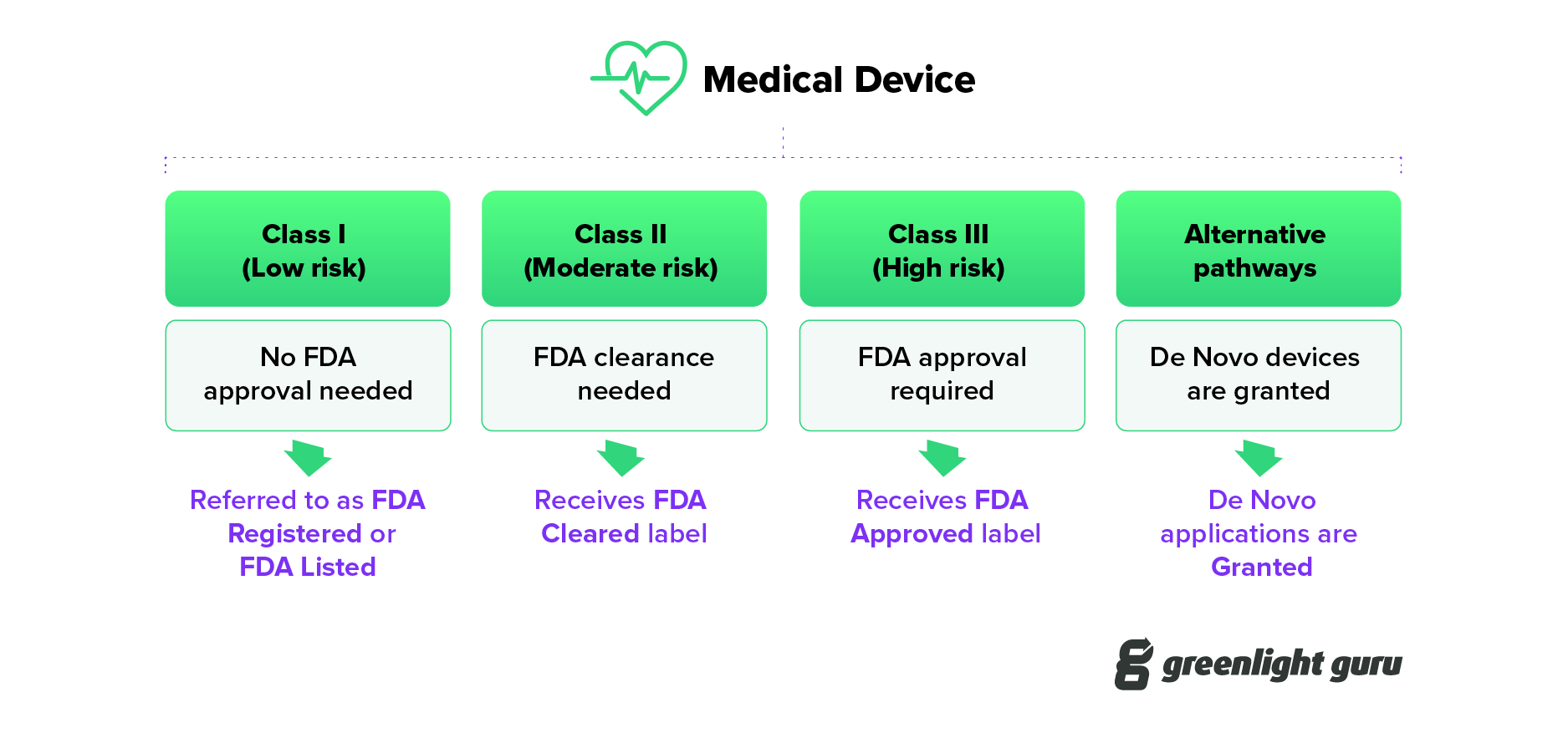
Ever wonder what FDA cleared vs approved vs granted actually mean? Learn the subtle yet important differences between these regulatory terms.
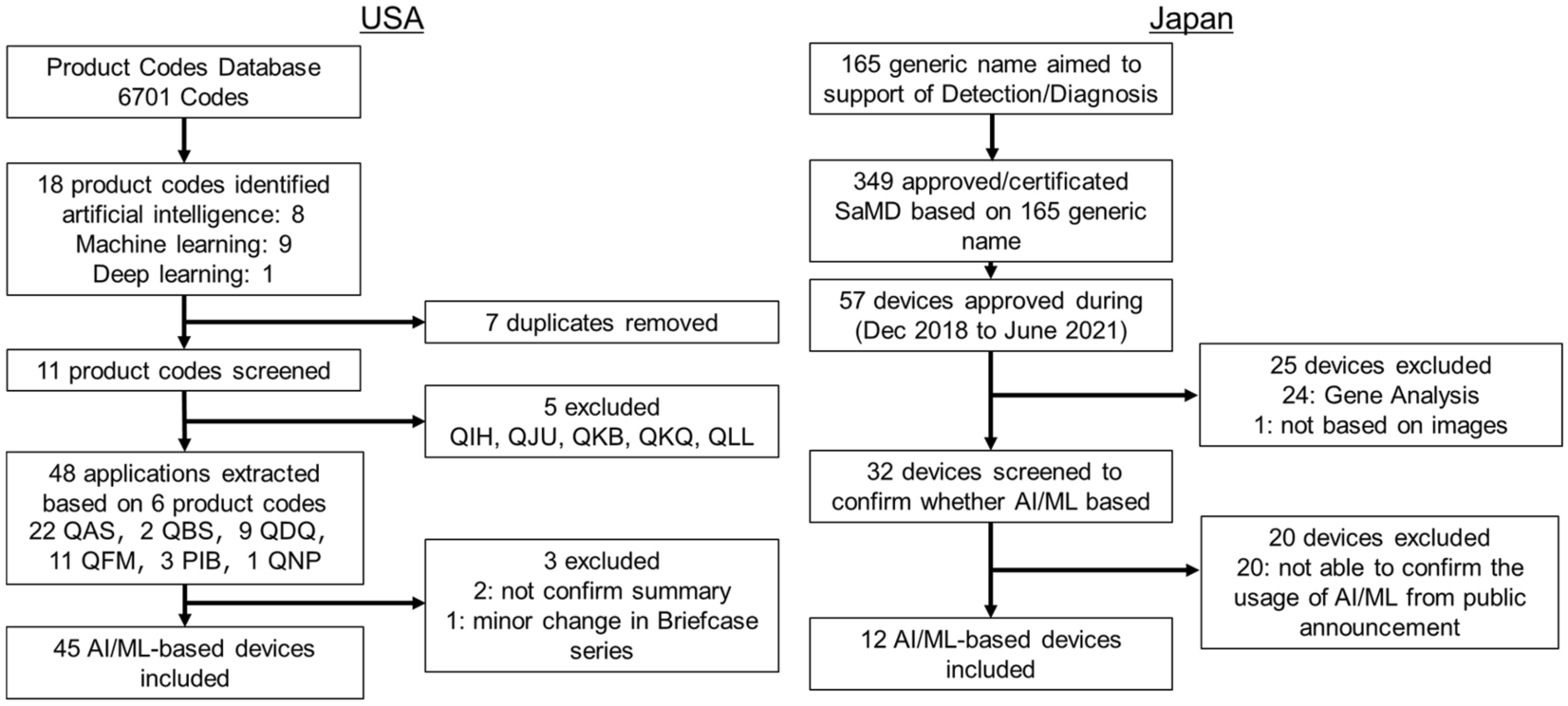
Systematic analysis of the test design and performance of AI/ML-based medical devices approved for triage/detection/diagnosis in the USA and Japan

Zebra Medical Vision Granted FDA 510k Clearance for AI Chest X-Ray Triage
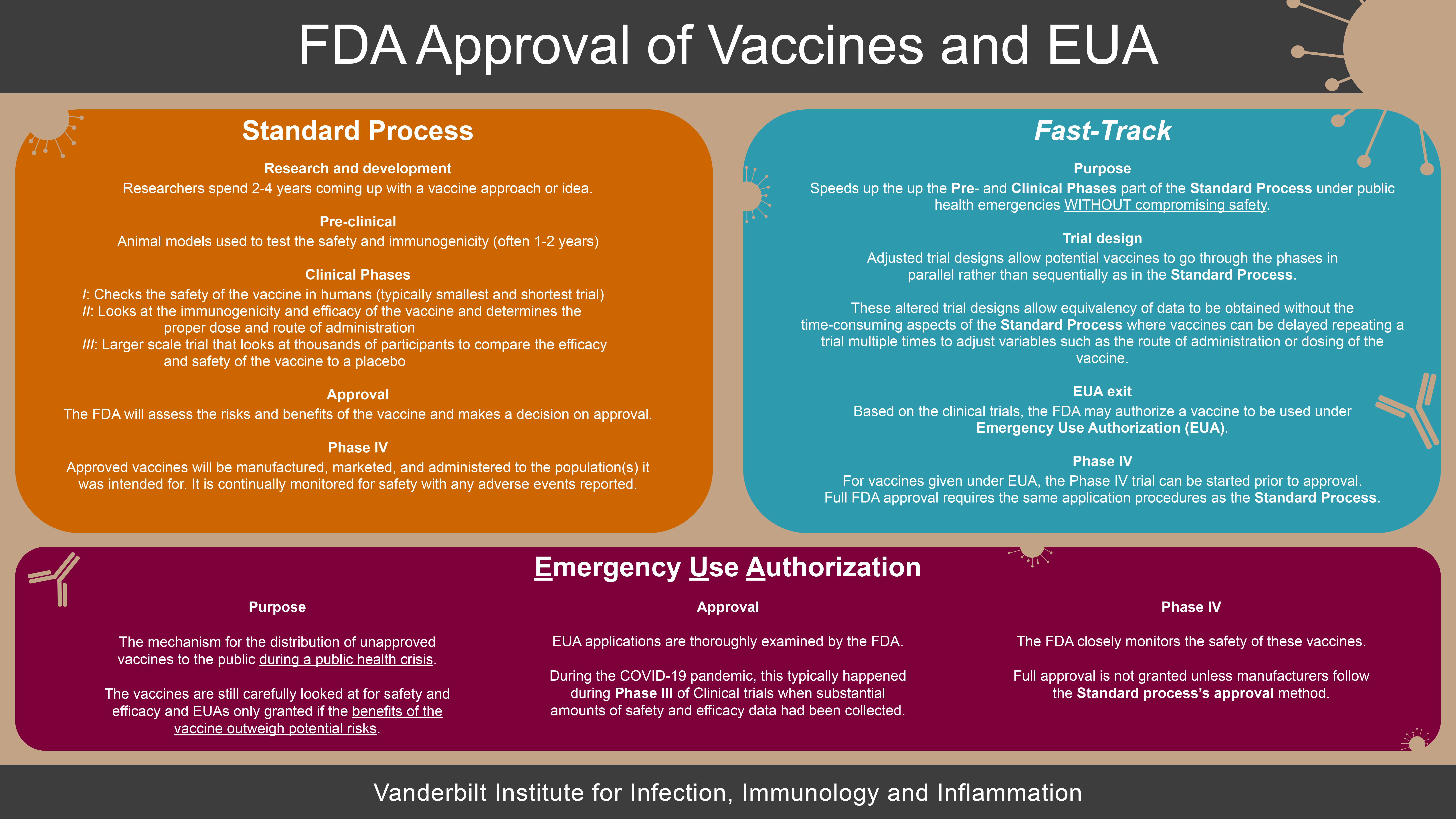
FDA Approval of Vaccines and EUA Infographic Vanderbilt Institute for Infection, Immunology and Inflammation

Medical device regulations, classification & submissions

CRSTG, Europe Edition

The Difference Between FDA Registered, Cleared, Granted, Authorized and Approved
%20(1).png)
What a Drug Recall Really Means

FDA Device Regulation: 510(k), PMA · Academic Entrepreneurship for Medical and Health Sciences
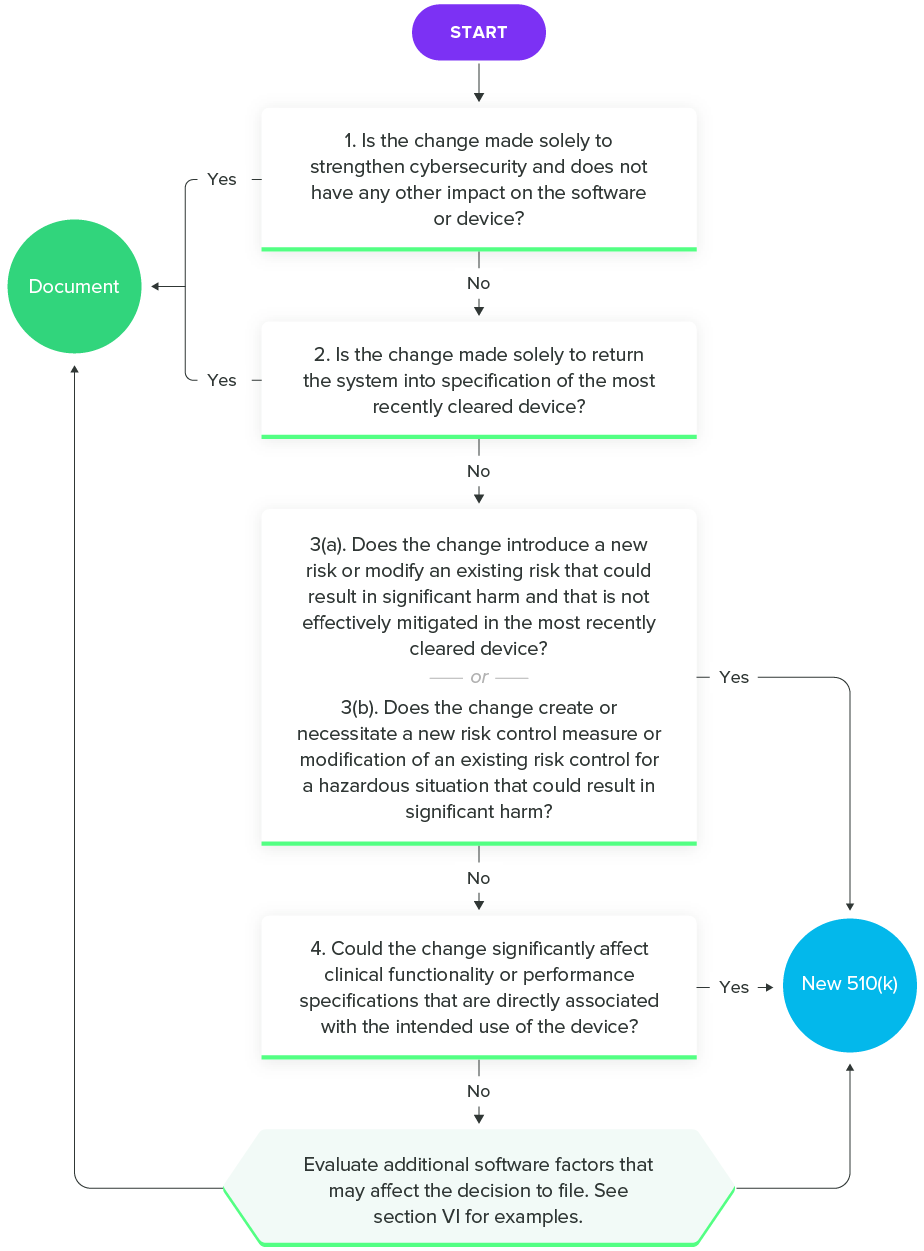
Software as a Medical Device (SaMD) - The Ultimate Guide

What is a Complete Response Letter?

FDA Listed vs. Cleared vs. Approved: What's the difference?

FDA listed, cleared, approved, granted - what IS the difference?
FDA Device Regulation: 510(k), PMA · Academic Entrepreneurship for Medical and Health Sciences

Yale researchers discover loophole in FDA medical device regulation - Yale Daily News

FDA Approved vs. FDA Cleared: Understanding Medical Device Regulations - Neuro20