Answer in Molecular Physics Thermodynamics for Neilmar #278440

thermodynamics - In the Statistical Mechanics Mark E. Tuckerman 4.4.7 - Physics Stack Exchange
Buy Introduction to Chemical Engineering Thermodynamics (The Mcgraw-Hill Chemical Engineering Series) on ✓ FREE SHIPPING on qualified

Introduction to Chemical Engineering Thermodynamics (The Mcgraw-Hill Chemical Engineering Series)

Physics Thermodynamics and Mol, PDF, Buoyancy
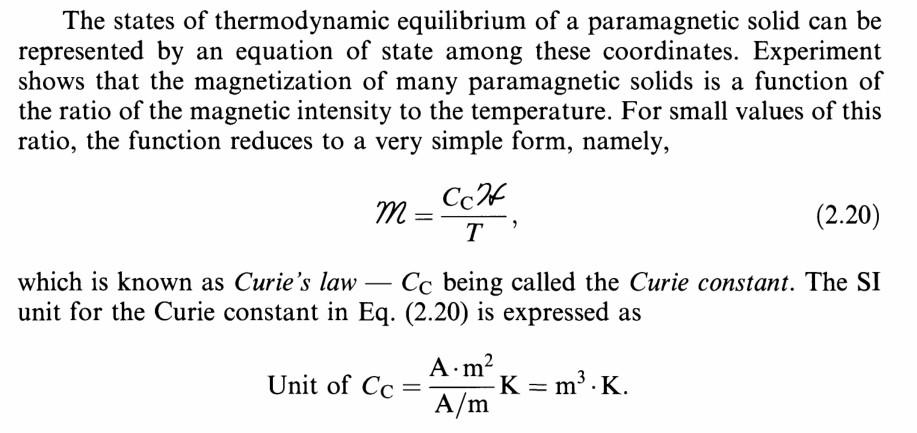
Solved Chapter 2 from Heat and Thermodynamic - Mark

Physics-1 - Molecular Physics and Thermodynamics, PDF, Heat Capacity

Physics-1 - Molecular Physics and Thermodynamics, PDF, Heat Capacity
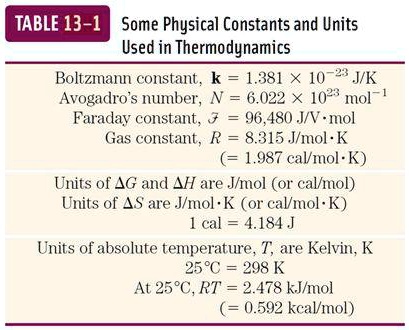
SOLVED: TABLE 13-1: Some Physical Constants and Units Used in Thermodynamics Boltzmann constant, k = 1.381 x 10^(-23) J/K Avogadro's number, N = 6.022 x 10^(23) mol^(-1) Faraday constant, F = 96,480

What is a Mole? - Avogadro's Number, Molar Mass & The Thermodynamic Limit (Daily Physics Ep1)

Fundamentals of Chemical Engineering Thermodynamics 1st Edition Themis Matsoukas Solutions Manual by a191653044 - Issuu

Molecular Thermodynamics - University Science Books

J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart - Introduction to Chemical Engineering Thermodynamics-McGraw-Hill Education (2018).pdf







