Solved Problem 3 (Phase changes) A 35 g ice cube at 0°C is

Answer to Solved Problem 3 (Phase changes) A 35 g ice cube at 0°C is

SOLVED: The specific heat capacity of ice is about 0.5 cal per gram per degree Celsius. Suppose it remains at that value all the way to absolute zero. Determine the quantity of

Temperature Change and Heat Capacity
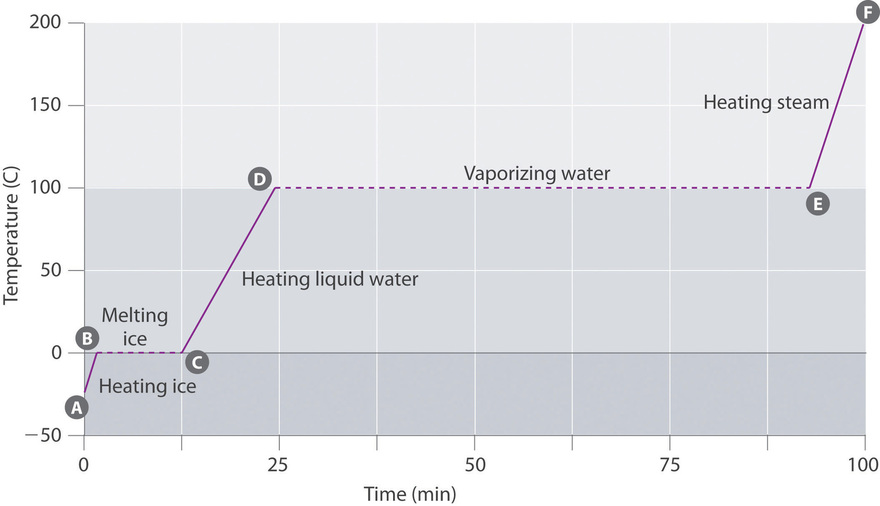
11.6: Phase Changes - Chemistry LibreTexts

14.3 Phase Change and Latent Heat

SOLVED: How much energy is required to change a 42g ice cube from ice at -11c to steam at 111c

Calculate the energy required to change a 10 g ice cube from ice at -10 deg C to steam at 110 deg C. ( c_{ice} = 2090 J/kg deg C, L_f =
:max_bytes(150000):strip_icc()/IceToSteam-58d96a7c3df78c516242a8cc.jpg)
Calculate Energy Required to Turn Ice Into Steam

14.3 Phase Change and Latent Heat – College Physics: OpenStax

Solved A 53.3-g ice cube is initially at 0.0°C. (a) Find the
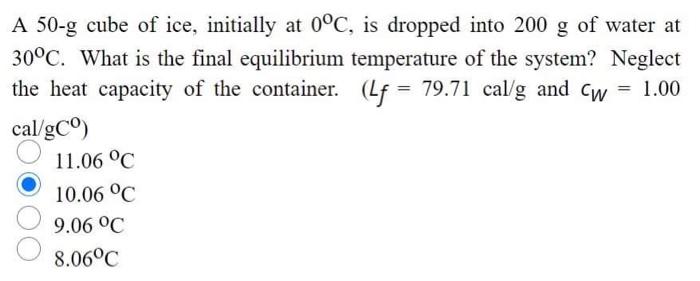
Solved A 50-3 cube of ice, initially at 0°C, is dropped into
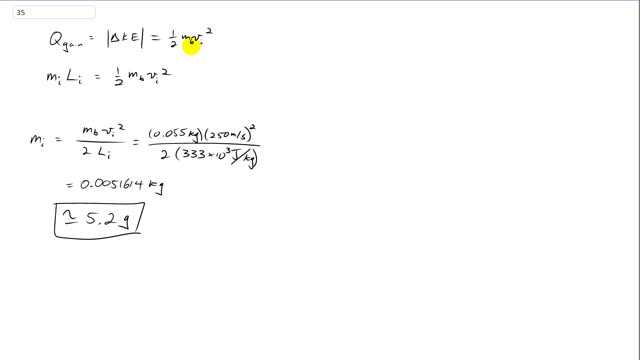
Giancoli 7th Edition, Chapter 14, Problem 35






/pub/media/catalog/product//2/1/215484551_cocoa_in.jpg?1697287899.963)
