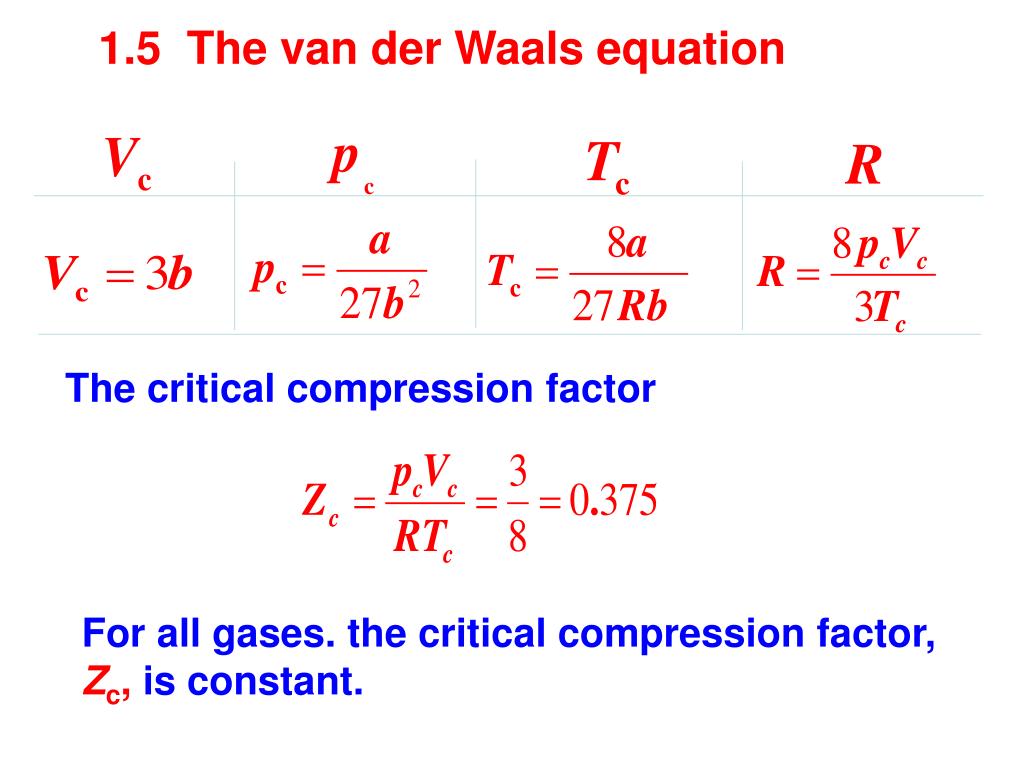
Solved The compression factor (Z) for a real gas can be

Description

Real gasses For an ideal gas, the compressibility factor Z = PV
Is z (compressibility factor) vs P (pressure) graph drawn by

Explain how the compression factor varies with pressure and

Real gas 1.molecules not always in motion (condense phase can be

Compressibility Factor of Gas Overview, Equation & Chart
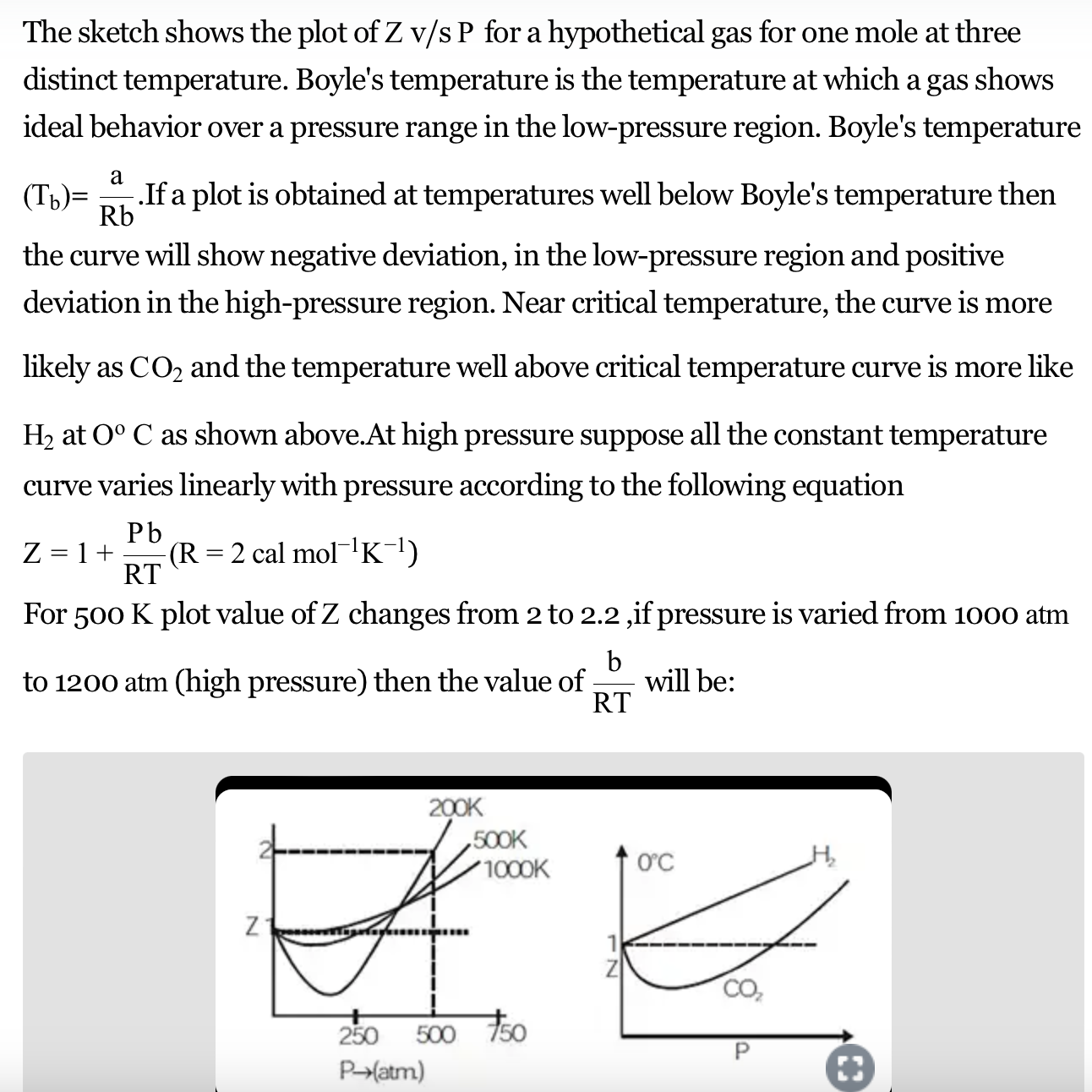
Solved The graph of compressibility factor (Z)v/sP for 1 mol

Thermodynamics - 3-7 Ideal Gas Equation with compressibility

gas laws - Graph of compressibility factor vs pressure when real
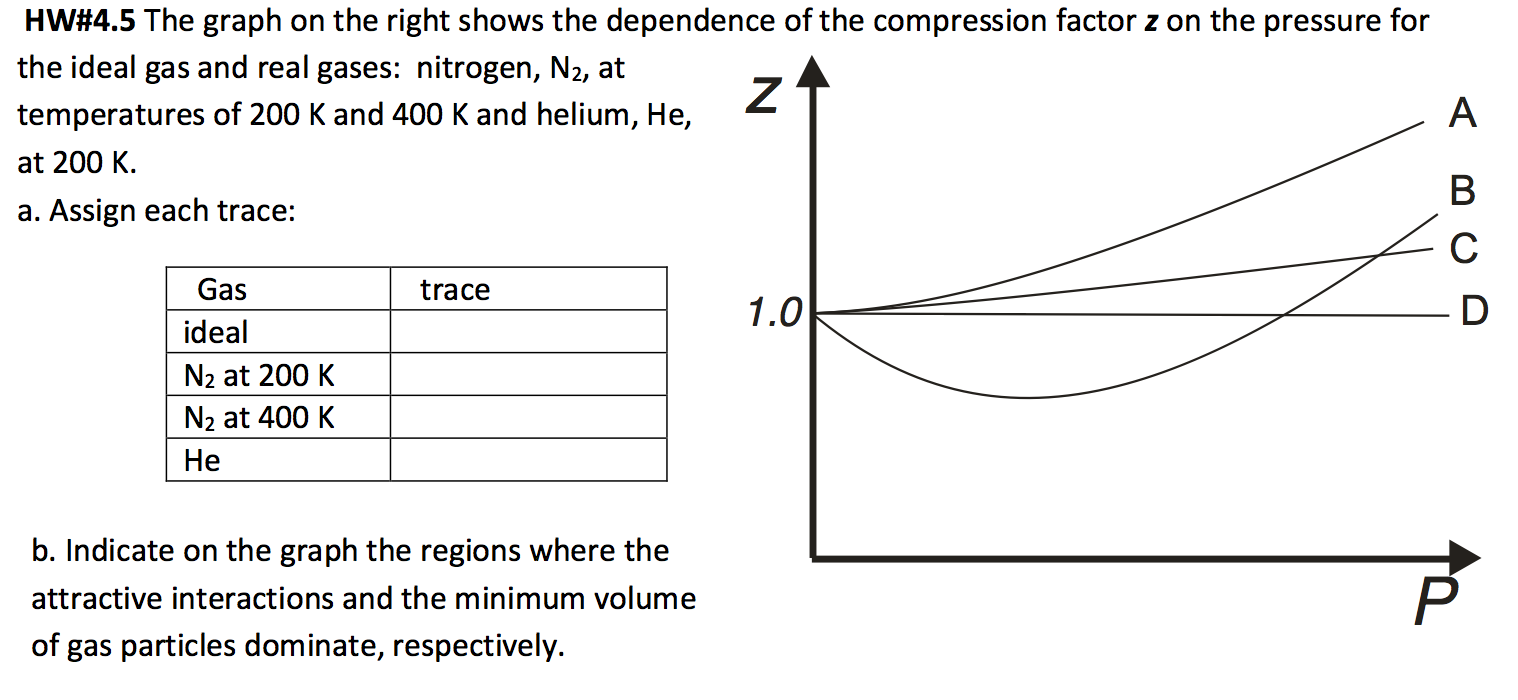
Solved HW#4.5 The graph on the right shows the dependence of

Solved RT B 2. The compressiblity factor for a gas is

physical chemistry - Is the compressibility factor smaller or

At high pressure, the compressibility factor 'Z' is equal toa
Related products
You may also like

Athleta farallon jogger in Mocha Latte size 8 In - Depop

Women Backless Sportsworkout Gym Underwear Shockproof Quick-drying Bra Wyelv

Young woman standing in jeans, black boots and lumberjack shirt. Side view. Full length studio shot isolated on white Stock Photo - Alamy

2021 Lava Thermal Bib Tights by Santini
$ 8.50USD
Score 4.8(688)
In stock
Continue to book
You may also like

Athleta farallon jogger in Mocha Latte size 8 In - Depop

Women Backless Sportsworkout Gym Underwear Shockproof Quick-drying Bra Wyelv

Young woman standing in jeans, black boots and lumberjack shirt. Side view. Full length studio shot isolated on white Stock Photo - Alamy

2021 Lava Thermal Bib Tights by Santini
$ 8.50USD
Score 4.8(688)
In stock
Continue to book
©2018-2024, farmersprotest.de, Inc. or its affiliates