The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT - Sarthaks eConnect
Description
The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT
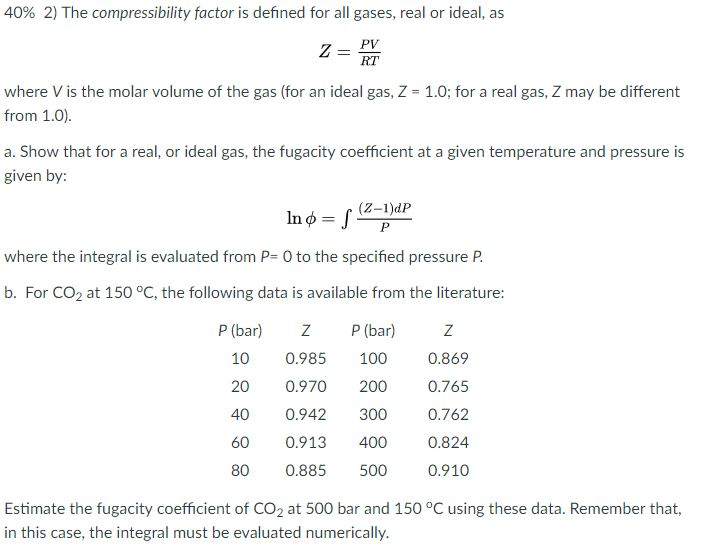
Solved 40% 2) The compressibility factor is defined for all

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

EngArc - L - Compressibility Factor

3.2 Real gas and compressibility factor – Introduction to
Simple Equation Real Gas Compressibility Factor Z

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

The compressibility factor a real gas high pressure is RT (b)1 po

The compressibility factor for a real gas is expressed by, z =1+

Solved We begin by showing that the compressibility factor
Related products
$ 12.50USD
Score 4.5(656)
In stock
Continue to book
$ 12.50USD
Score 4.5(656)
In stock
Continue to book
©2018-2024, farmersprotest.de, Inc. or its affiliates





