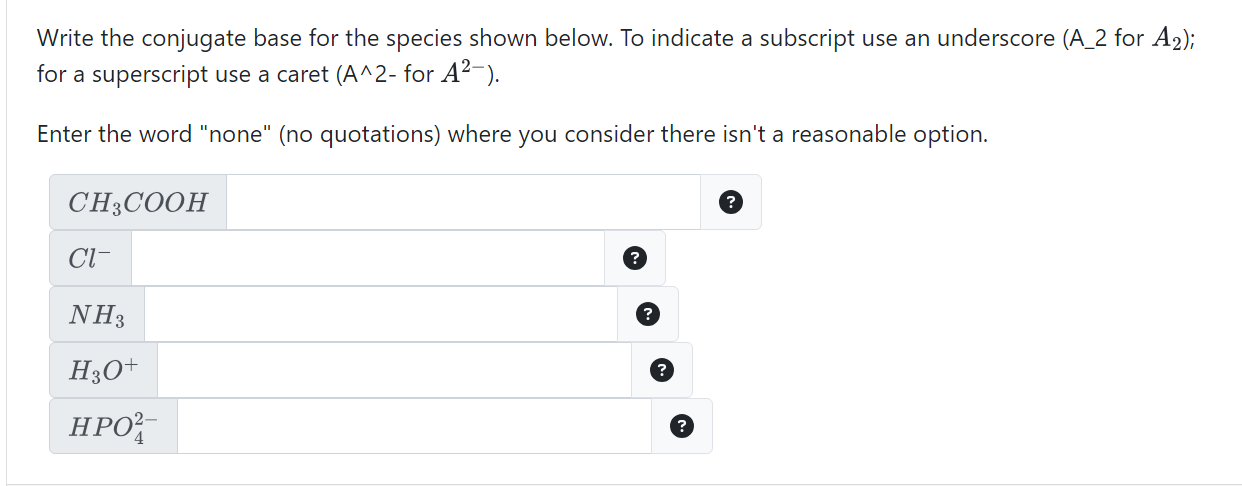
Solved Write the conjugate base for the species shown below

Answered: Part C H2PO3 Express your answer as a…
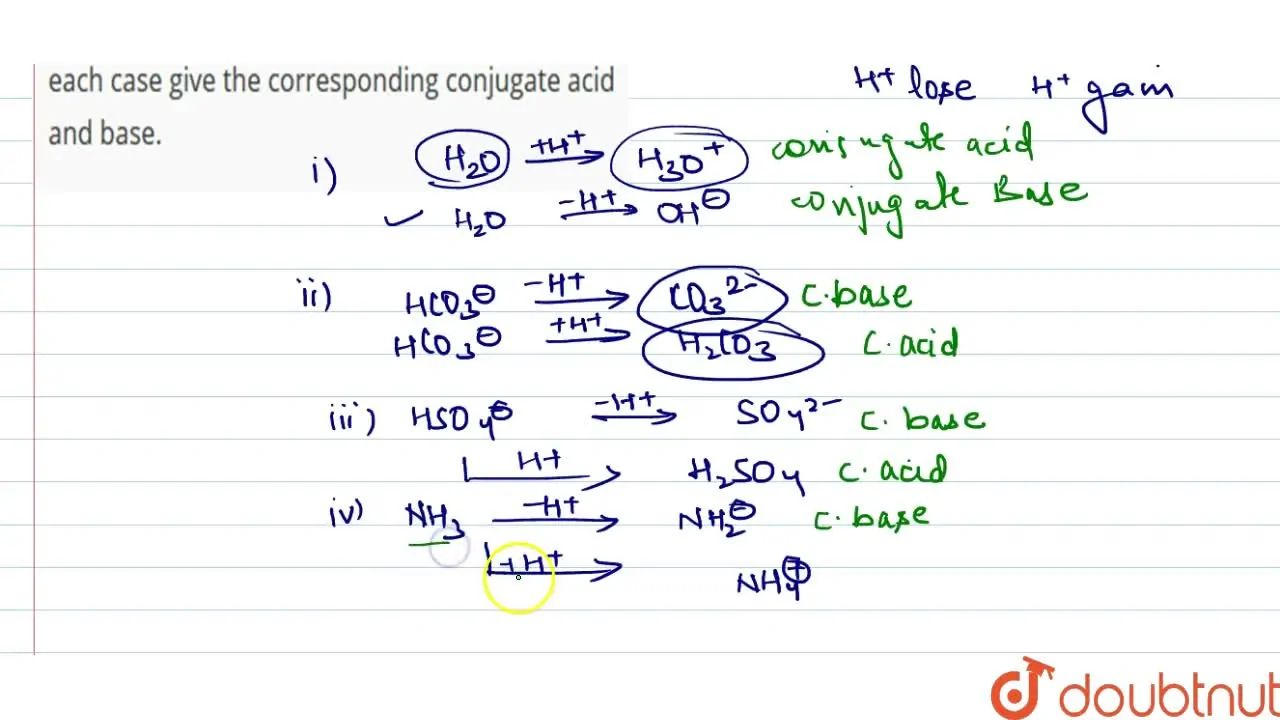
The species: H(2)O, HCO(3)^(Θ), HSO(4)^(Θ) and NH(3) can act both as B

For each conjugate acid-base pair, identify the first species as an acid or a base and the second species as its conjugate acid or base. In addition, draw Lewis structures for each

Solved 1. Give the conjugate base of each of the following

Caffeine (C8H10N4O2) is a weak base with a pKb of 10.4. Calculate
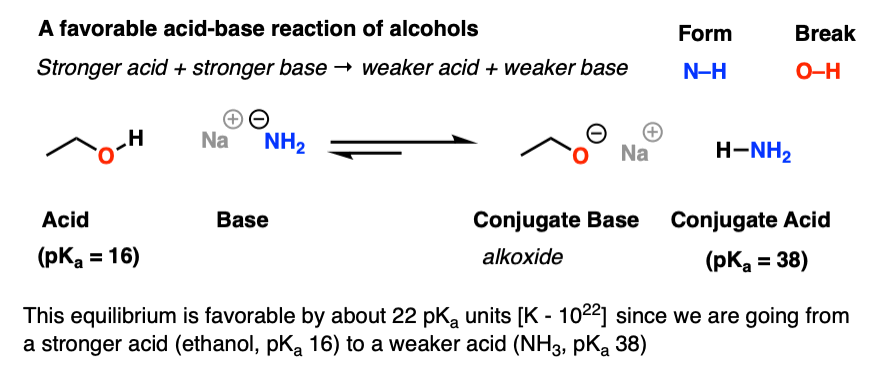
Acidity and Basicity of Alcohols – Master Organic Chemistry
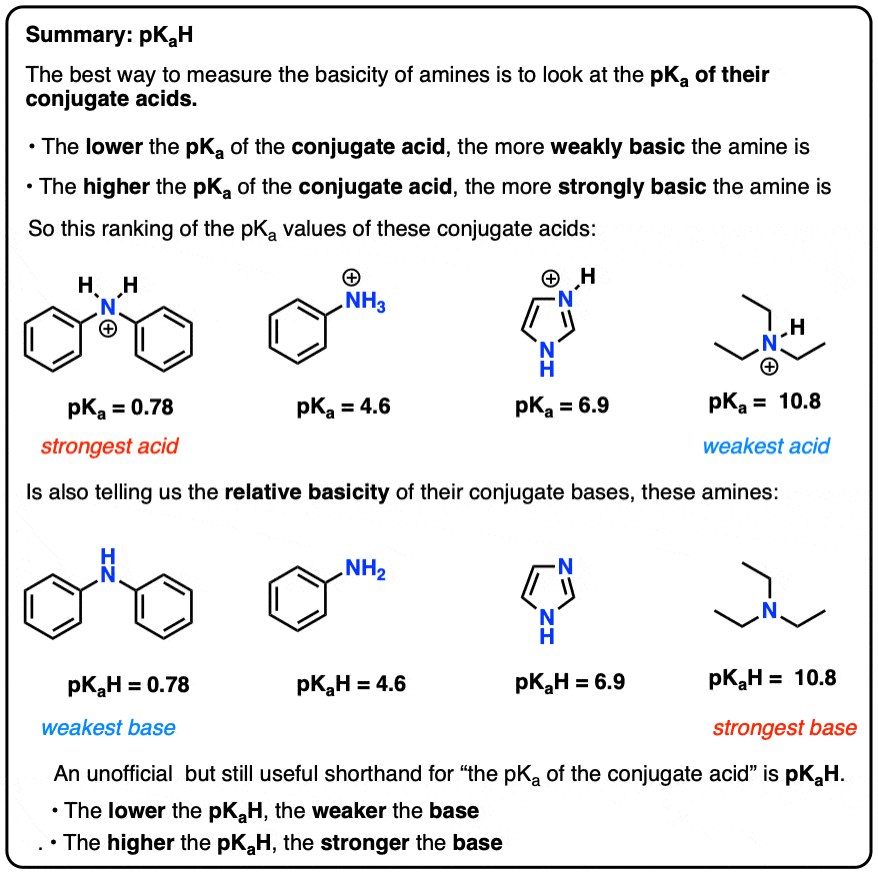
Amine Basicity Is Measued By The pKa Of Its Conjugate Acid (pKaH)

Complete a net ionic equation for each proton-transfer reaction using curved arrows to show the flow of electron pairs in each reaction. Label the original acid and its conjugate base; then label
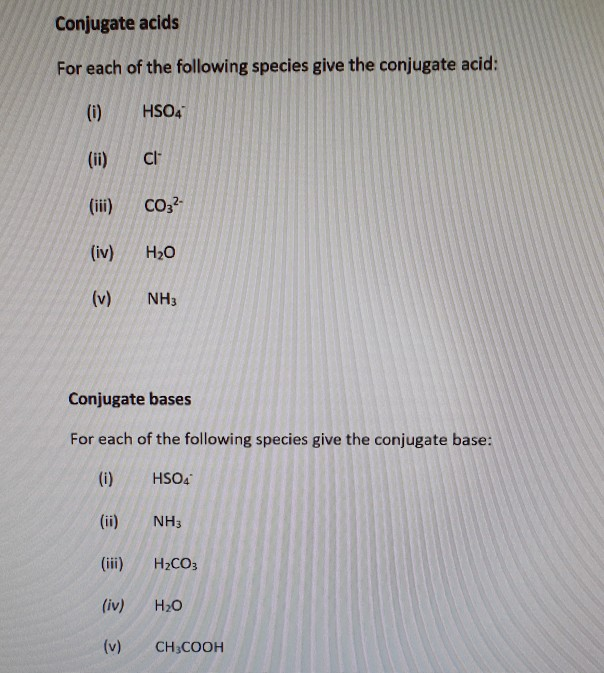
Solved Conjugate acids For each of the following species

How to Choose an Acid or a Base to Protonate or Deprotonate a Given Compound - Chemistry Steps

Solved 4) Indicate the conjugate acid or base of the species






