The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1

Calculate the entropy change for the conversion of2 moles of liquid wa

The entropy change when `36g` of water evaporates at `373 K` is `:-` `(DeltaH=40.63(KJ)/(mol))`

25. The enthalpy of Vaporization of benzere is r.3 kJ/mol its boiling point of suche copy change in the train of Vapour tout its boiling point is ---- 11100 2) +100 B

Phase change material-based thermal energy storage - ScienceDirect

The entropy change when 36 g of water evaporates 373 K is (AH = 40.63 kJ mol): (A) 218 JK- (B) 150 JK-1 (C) 118 JK-1 (D) 200 JK-1 (C) 118 JK (0) 200 JK
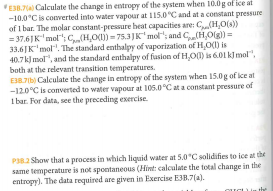
E Calculate the change in entropy of the system when

50. Ir Water vapour is assumed to be a perfect gus, molar enthalpy change vapourisation of 1 mole of water 1 bar and 100° C is 41 mol Calculate the internal energy

The entropy change involved in the conversion of 1 mole of liquid water at 373 K to vapour will be:Given: H vap =2.257 kJ / gA. 150 JK 1 mol 1B. 130.6

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is

Answered: 6.14. Estimate the entropy change of…

⏩SOLVED:The entropy change when 36 g of water evaporates at 373 K…

calculate the entropy change involved in conversion of one mole (18g) of solid ice at 273 K of liquid water - Chemistry - Thermodynamics - 9709217

What is the correct method to convert volumetric flow rate of mixture to mass flow rate ? : r/thermodynamics
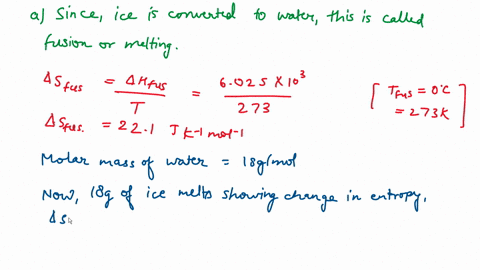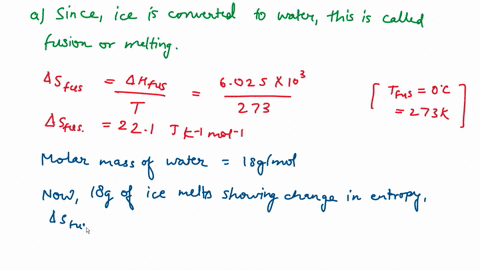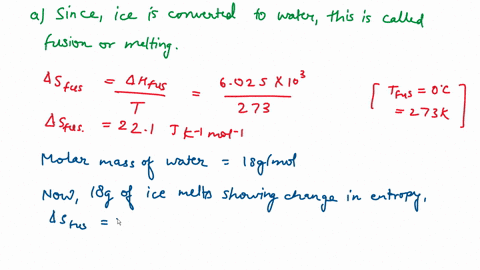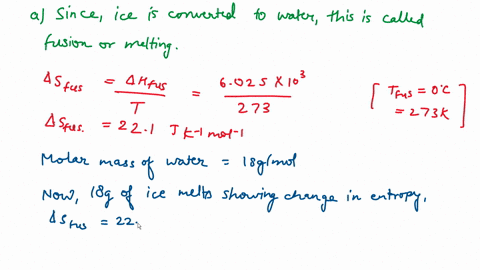
⏩SOLVED:Calculate the entropy change for the conversion of…







