Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt


Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Properties of Gas Manik
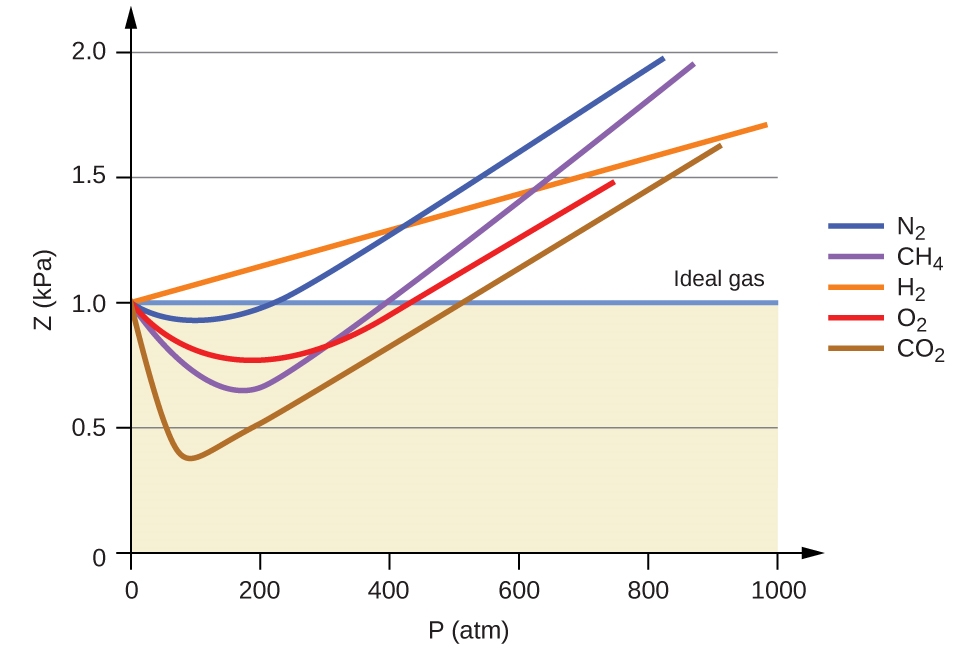
2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Properties of gases extended oct 2020

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Compressibility Factor of Gas Overview, Equation & Chart
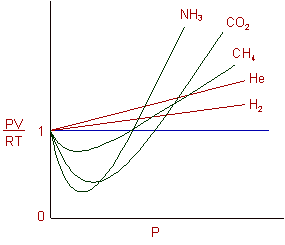
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

3.3: Real gas and compressibility factor - Engineering LibreTexts
Why compressibility factor of areal gas is greater than unity at

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i) What is the value of Z for an

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Properties of Gas Manik
Solved The compressibility factor Z = PVm/RT is used to