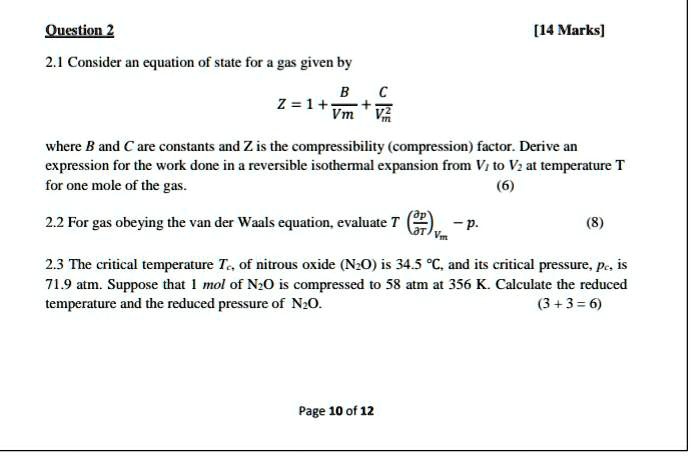
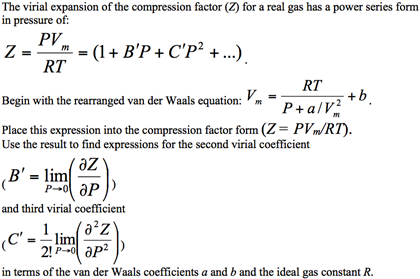
Solved The virial expansion of the compression factor (Z)

PDF) Chemistry 360 Problem Set 2 Solutions
A New Method for Estimating Compressibility Factors of Natural
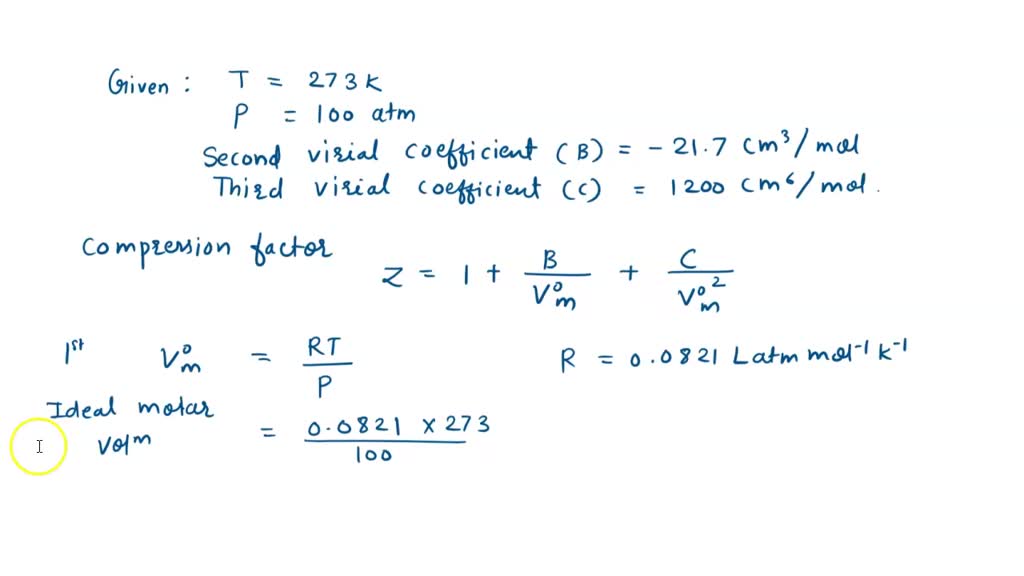
SOLVED: At 273 K, measurements on argon gave B = -21.7 cm^3/mol and C = 1200 cm^6/mol^2, where B and C are the second and third virial coefficients in the expression of

Third Virial Coefficient of the Equation of State - an overview

PDF) Theoretical Assessment of Compressibility Factor of Gases by Using Second Virial Coefficient

1.7: Connecting the van der Waals and the viral equations: the

At 273k measurements on ar gave b=-21.7cm3mol-1 and c=1200cm6mol-2
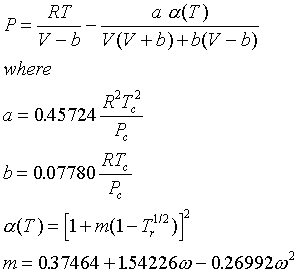
Thermodynamic Models

At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{
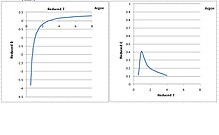
Virial expansion - Wikipedia

Answered: Consider the van der Waals equation of…

I need help with question 3: a,b,c, i'm stuck and

Virial Equation of State2, PDF, Physical Chemistry

Solved (Triple-Play Bonus) For a certain gas, the