The compressibility factor Z for an ideal gas will be

The compressibility factor Z for an ideal gas will be

The value of compressibility factor (`Z`) for an ideal gas is

Physical Chemistry The Compression Factor (Z) [w/1 example]

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
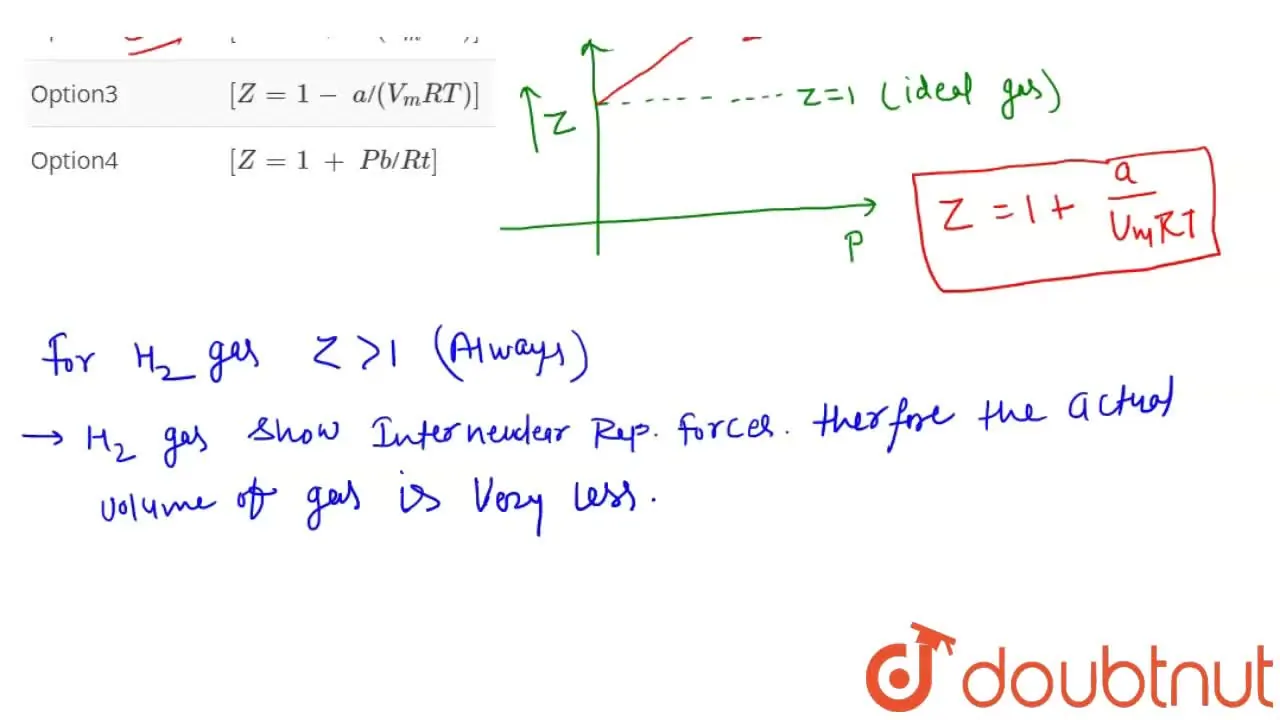
Compressibility factor (Z) for H2 (g) at STP is

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!
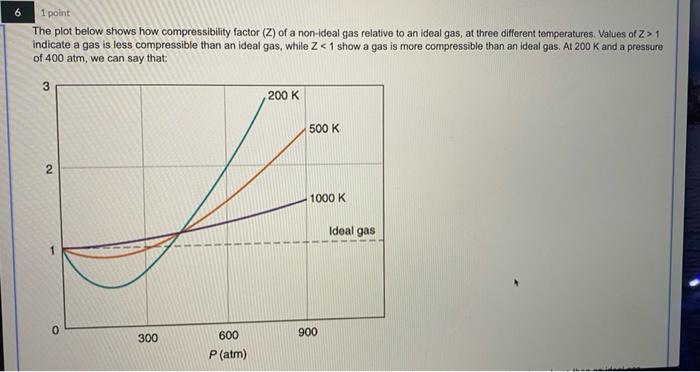
Solved 6 1 point The plot below shows how compressibility
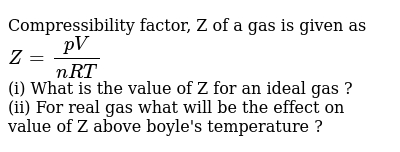
Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

68. The compressibility factor (z) an ideal gas is equal to which of the following values? (A) Zero (B) Less than one (C) Equal to one
Nonideal Gas Behavior - Course Hero

ERS TYPEnThis section contains quostions where the answer to each
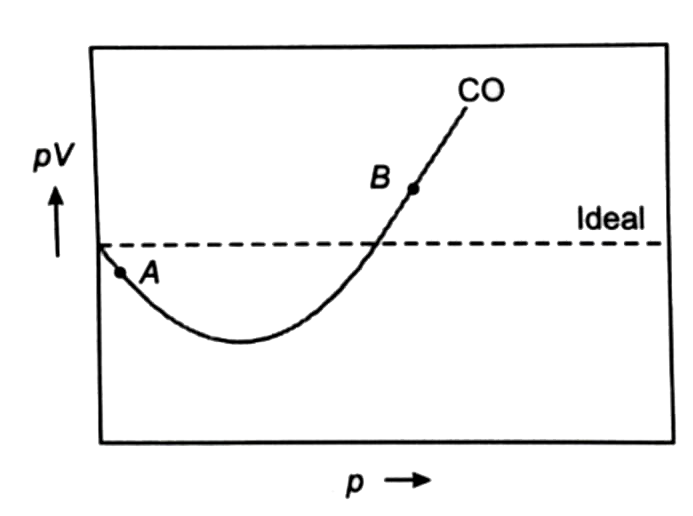
For Co, isotherm is of the type as shown. Near point A, compressibilit

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure






