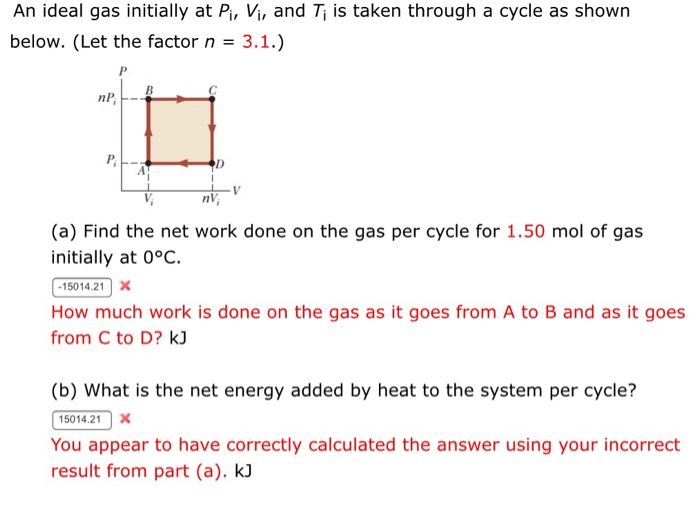

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of

An ideal gas has initial volume V and pressure p. If the volume of gas is doubled during expansion, then minimum work will be done in which thermodynamic process ?A. Isobaric processB.

⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

Solved] If one mole of an ideal gas at (P1, V1) is allowed to expand
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n 3.6.) nP; P; nV; (a Find the net work done

An ideal gas is taken around the cycle `ABCA` shown in `P - V` diagram. The net work done by the
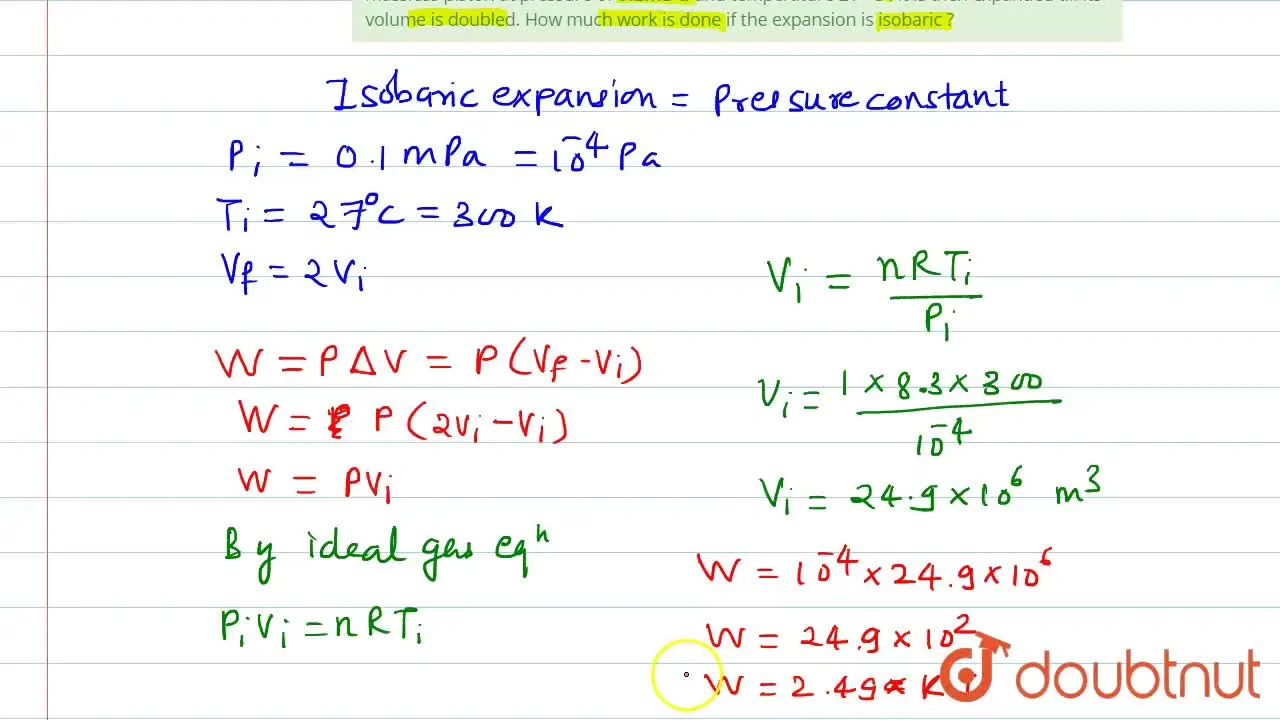
Marathi] One mole of an ideal gas is initially kept in a cylinder wit
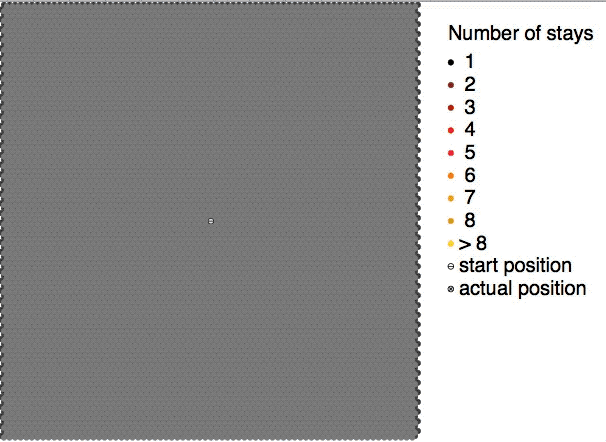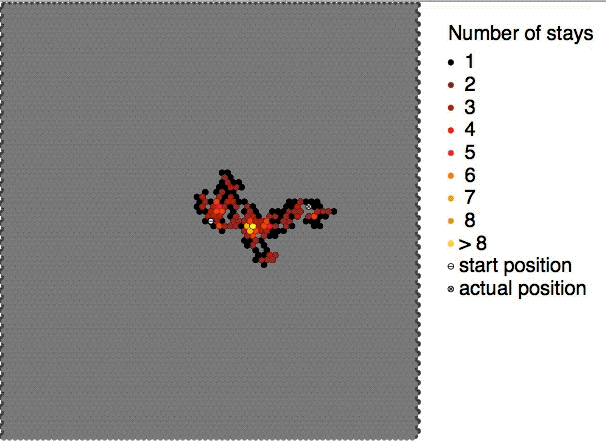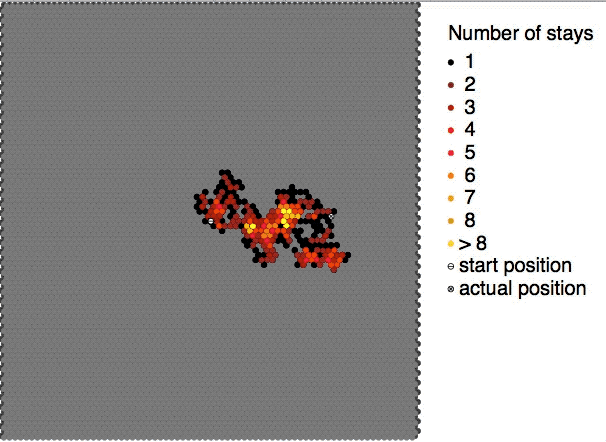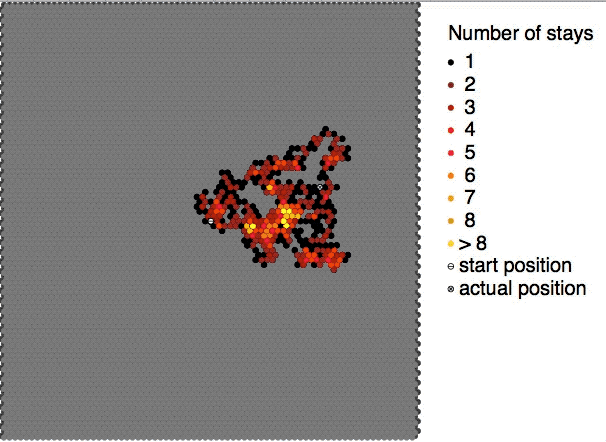
Brownian motion - Wikipedia
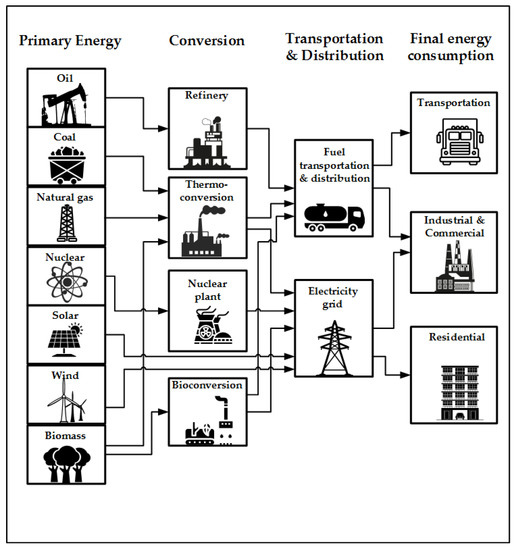
Processes, Free Full-Text
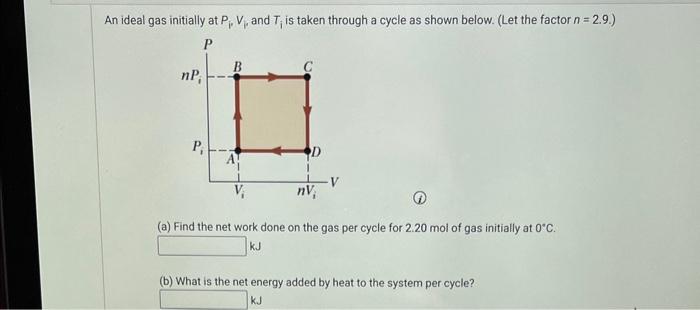
Solved An ideal gas initially at Pi,Vi, and Ti is taken
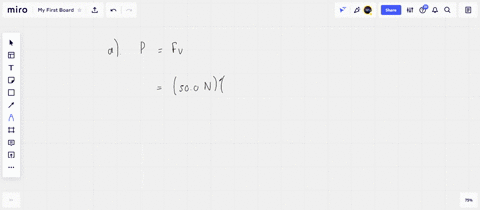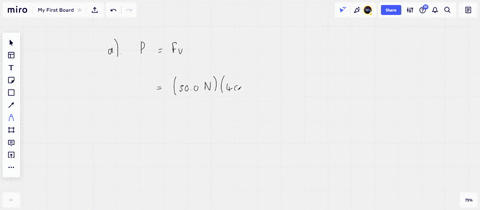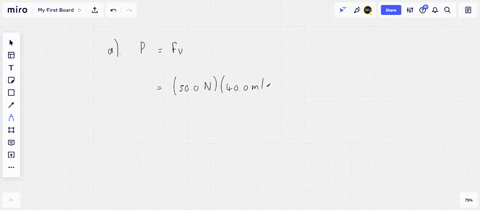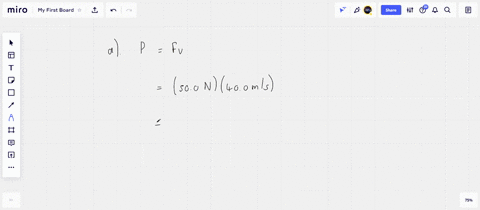
⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…







