physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Description
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

physical chemistry - Pressure vs volume plot for real gas and

Energies, Free Full-Text
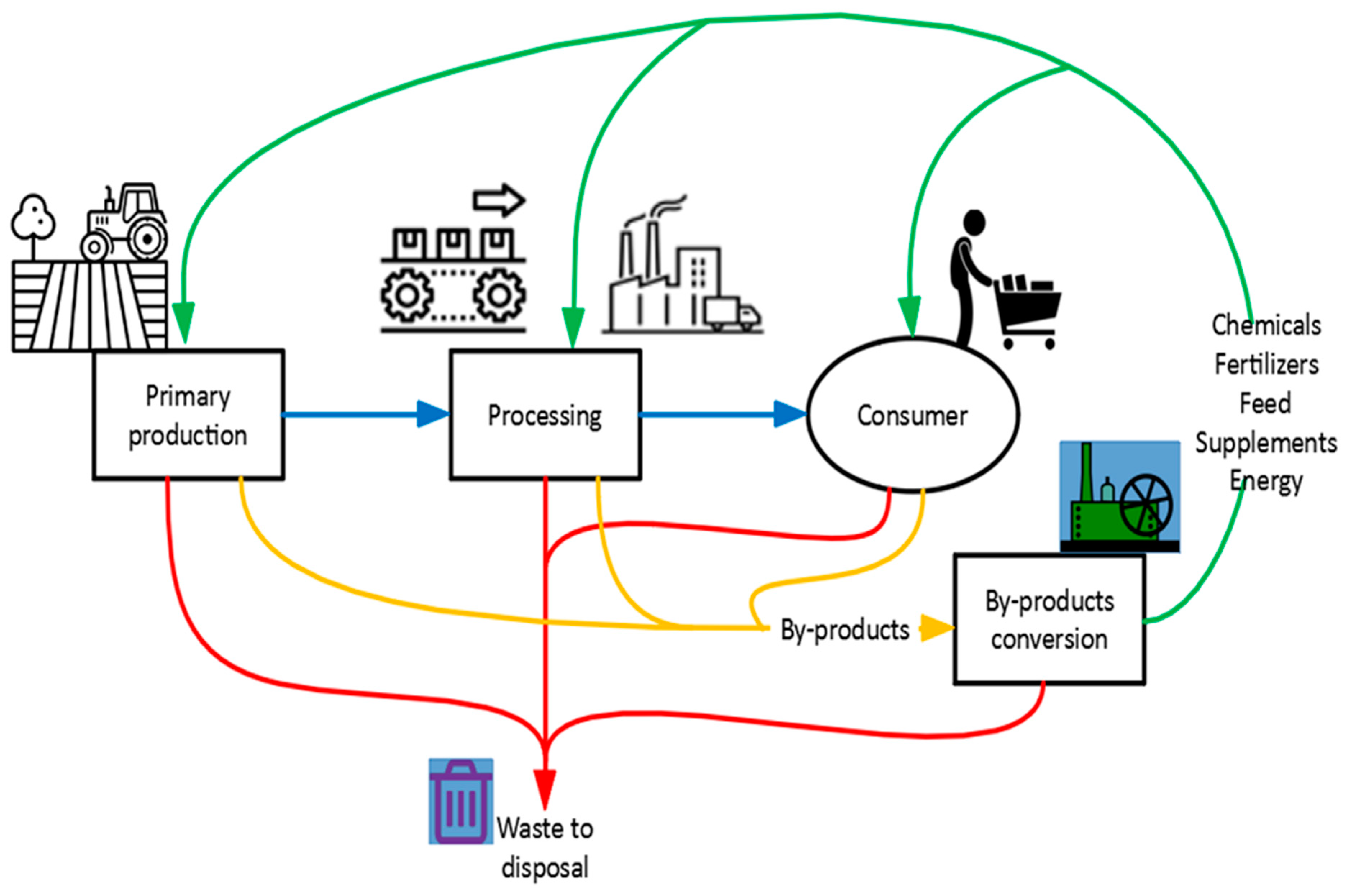
Energies, Free Full-Text
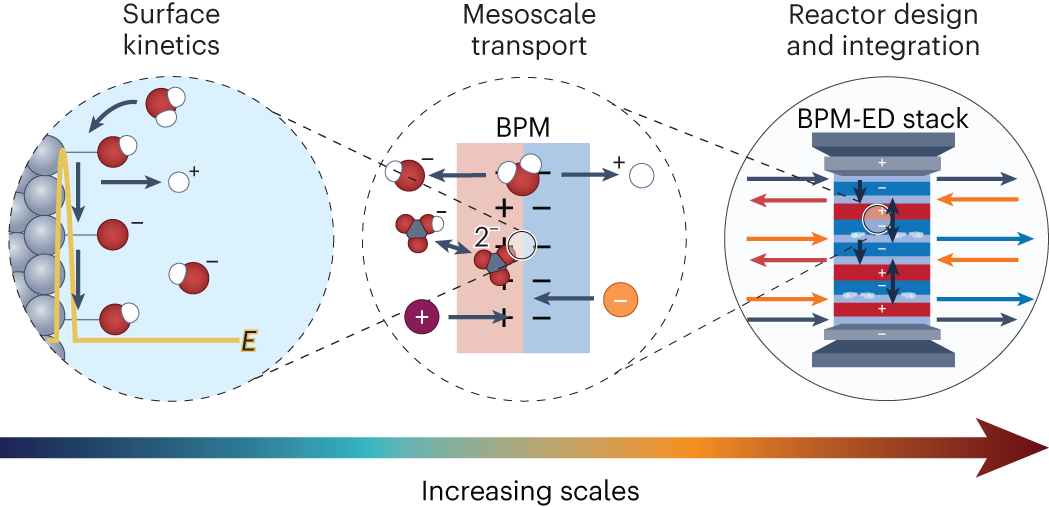
Multi-scale physics of bipolar membranes in electrochemical
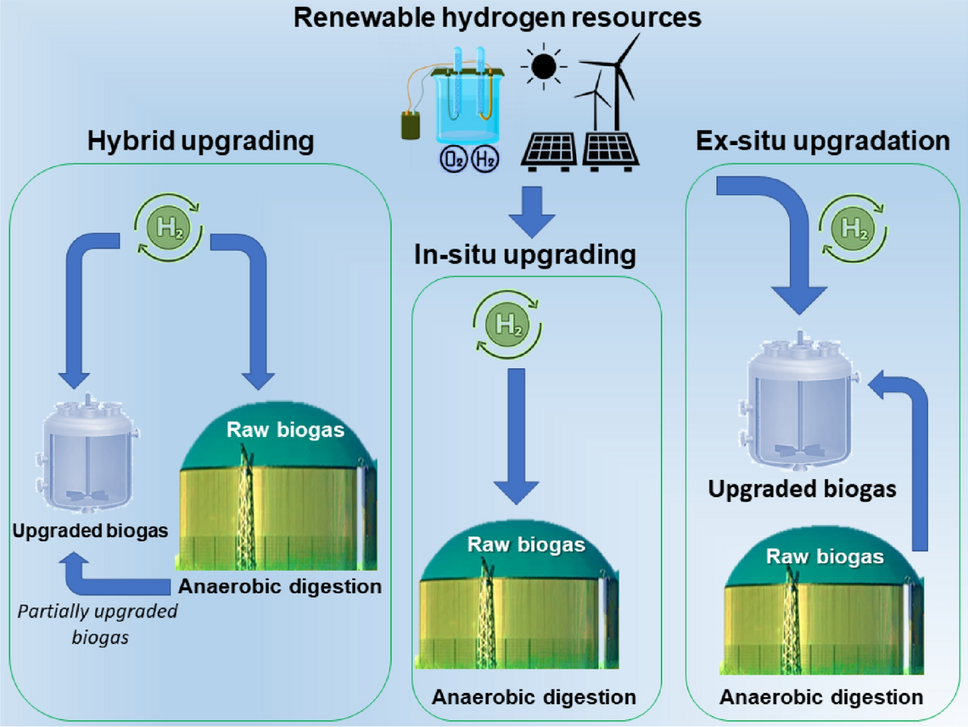
Integration of biogas systems into a carbon zero and hydrogen

physical chemistry - why is the pressure exerted by ideal gas on

physical chemistry - Is the compressibility factor smaller or
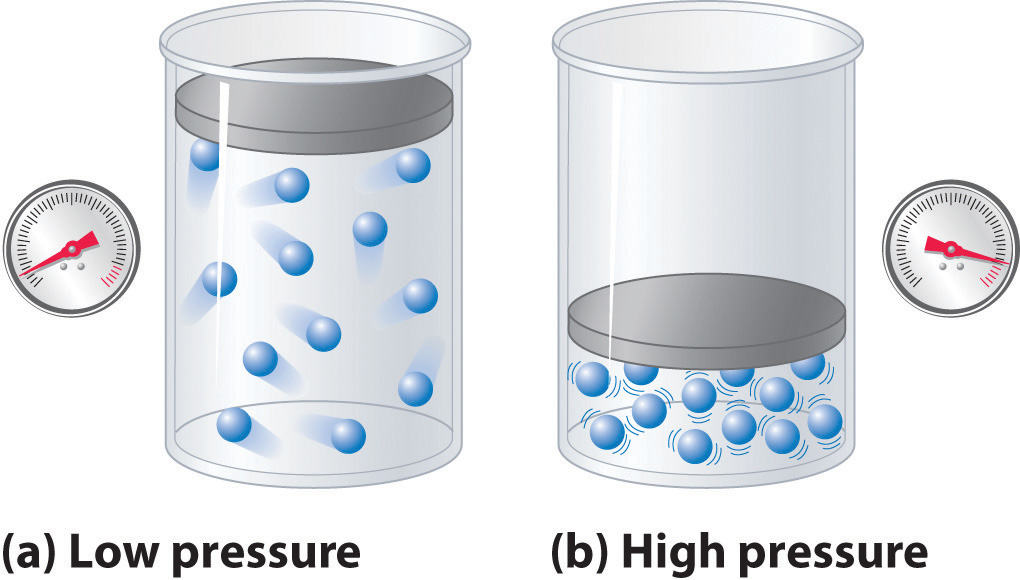
The Behavior of Real Gases
Applied Sciences, Free Full-Text
Related products
$ 15.50USD
Score 4.5(168)
In stock
Continue to book
$ 15.50USD
Score 4.5(168)
In stock
Continue to book
©2018-2024, farmersprotest.de, Inc. or its affiliates






