20.If Z is a compressibility factor, van der Waals equation at low
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

16.4: The Law of Corresponding States - Chemistry LibreTexts

Deviations from ideal gas behaviour, intermolecular forces, Van

012 IfZ is a compressibility factor, van der Waals equation low pressure can be written as: [2014] RT I-끔 (C) Z-I+ Z=1+ (B) Ζ=I.RT (D) Z=l- _ pb VRT
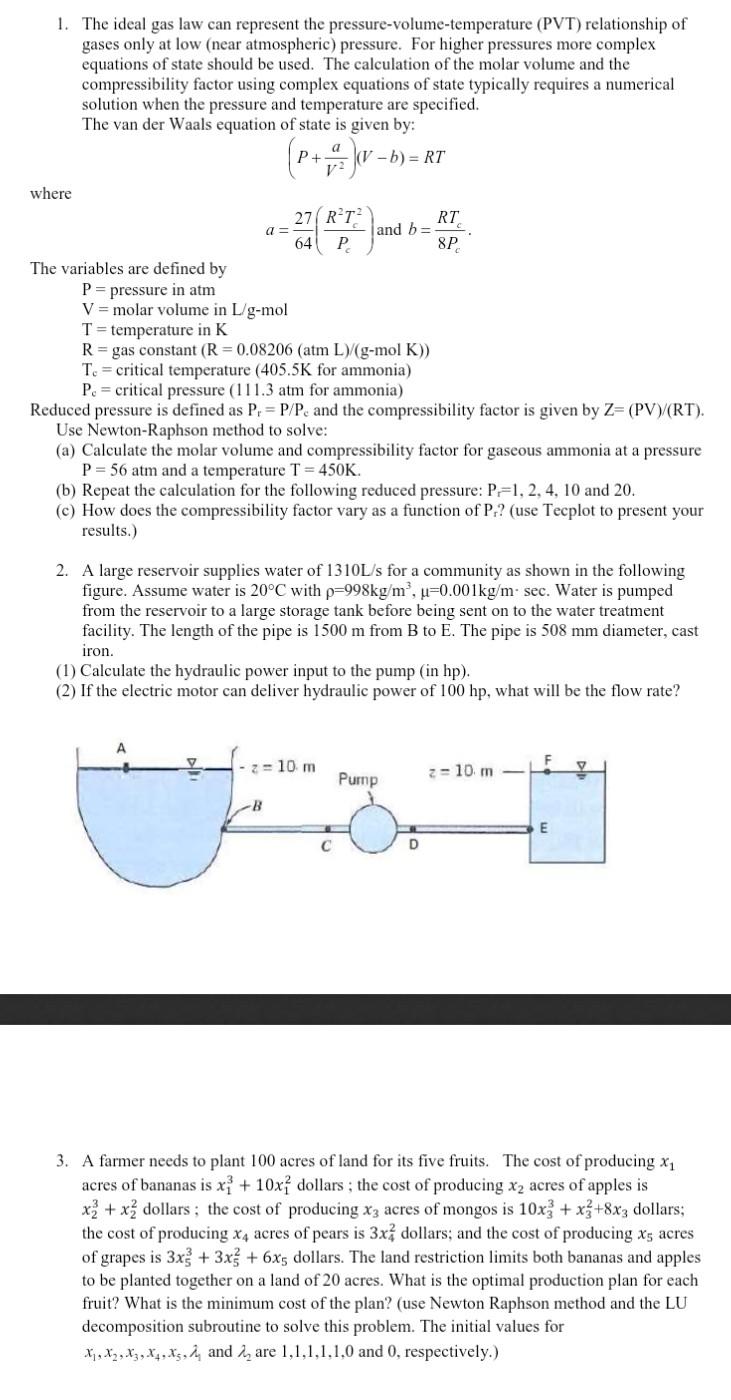
Solved Can you solve the problem and add fortran code for

Compressibility factor (gases) - Citizendium
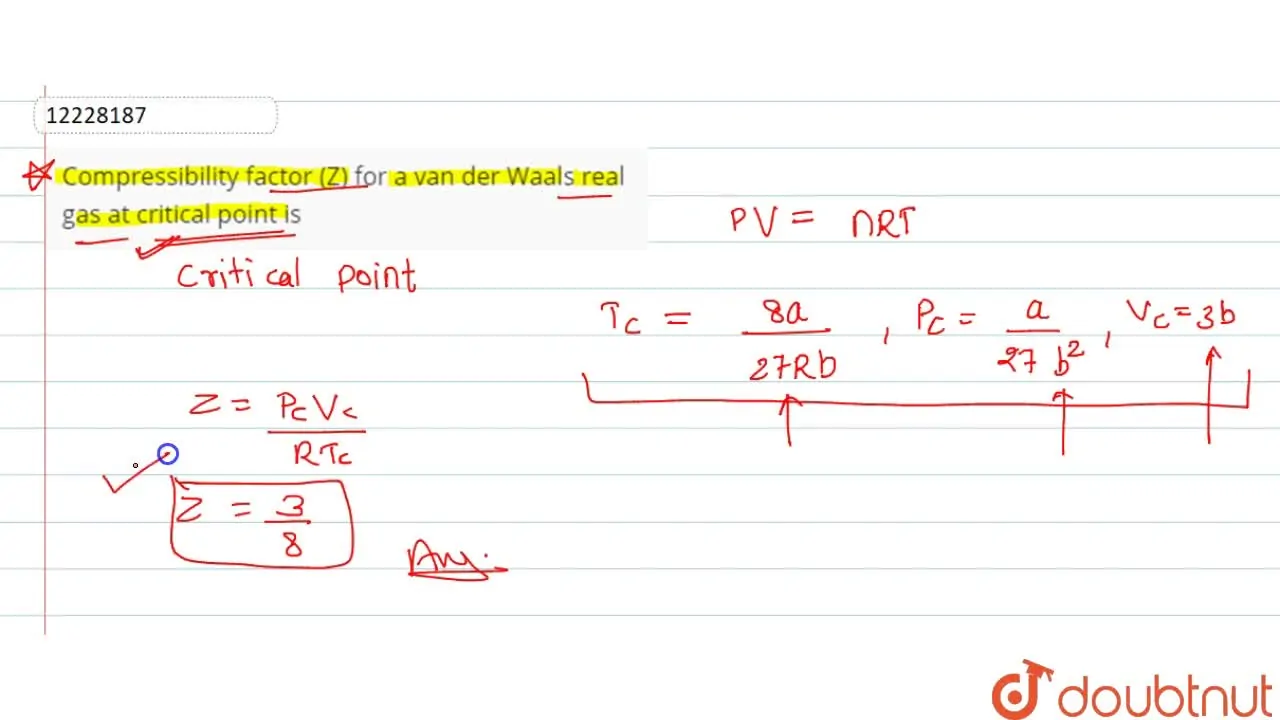
Compressibility factor (Z) for a van der Waals real gas at critical po

Solved Problem 1: Molar Volume and Compressibility Factor

The given graph represent the variations of Z Compressibility
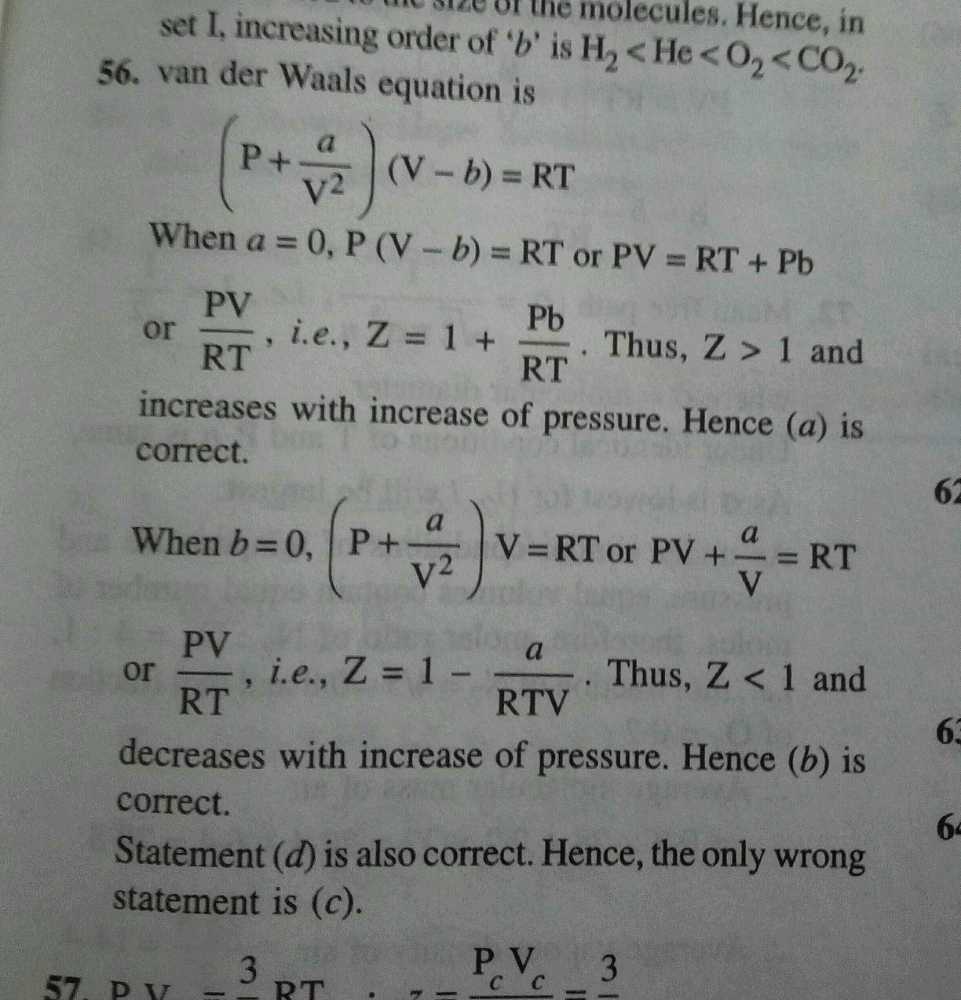
The given graph represents the variation of Z (compressibility factor

Van der Waals equation, when pressure correction is ignored, one

At a high pressure, the compressibility factor (Z) of a real gas is us
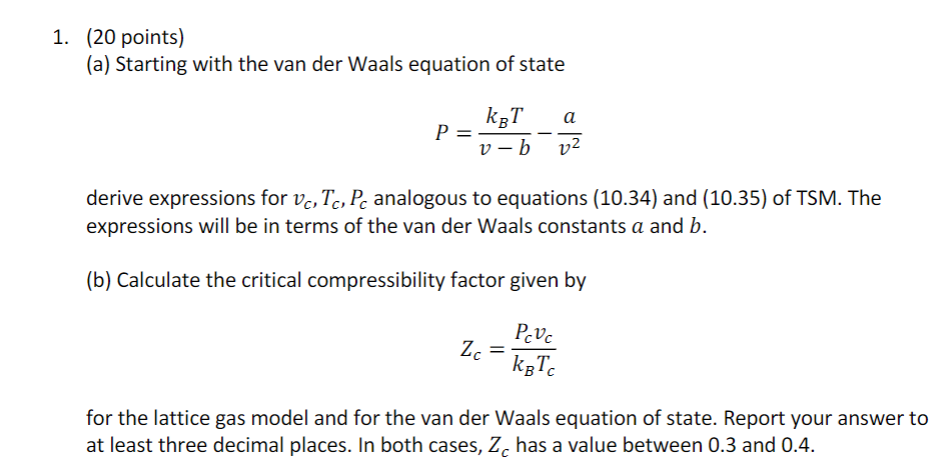
Solved (20 points)(a) Starting with the van der Waals

physical chemistry - Why do some gases have lower value of Z for a










