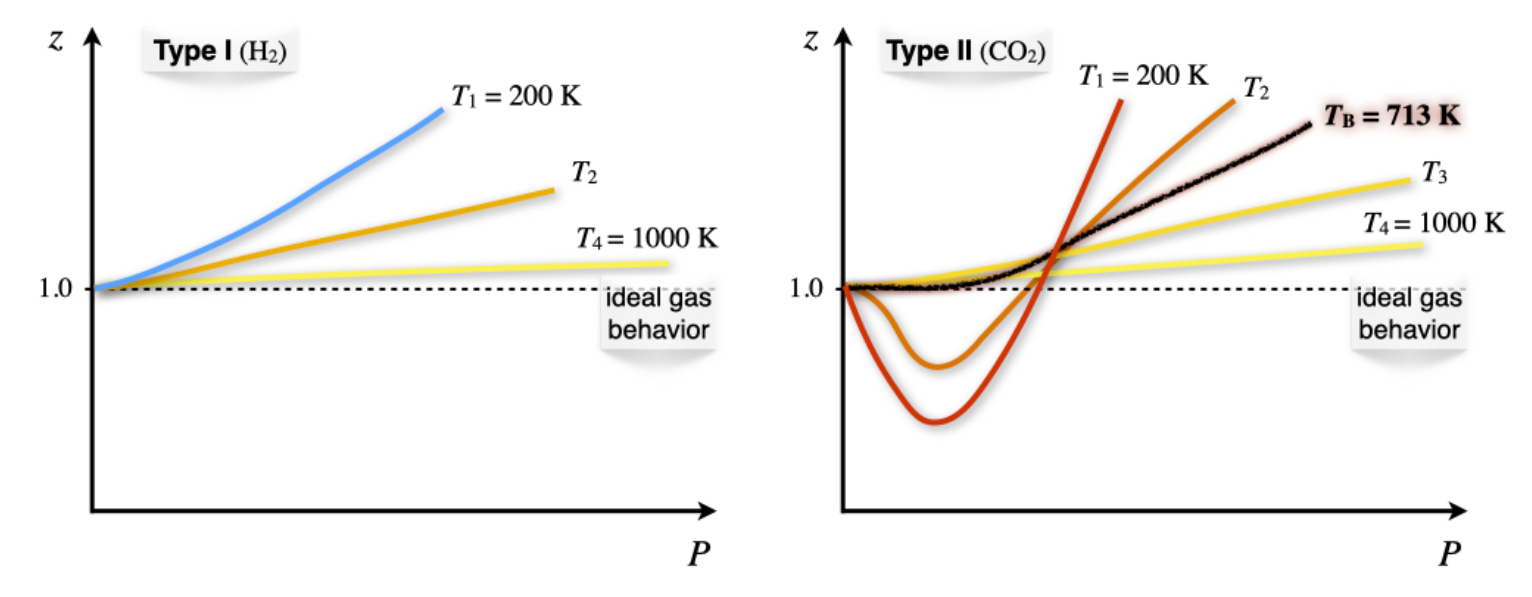
Compressibility factor Z = PV / nRT is plotted against pressure as

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
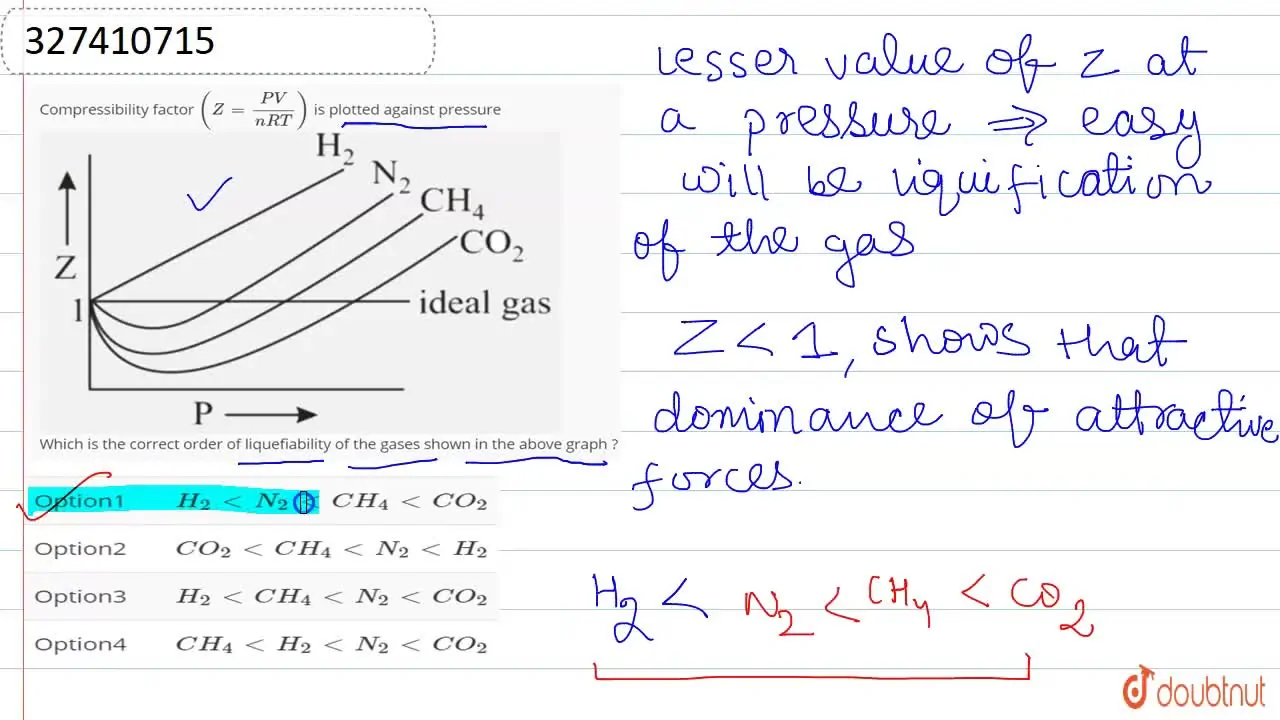
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Deviation of Real Gases from Ideal Gas Behaviour - Chemistry for ACT PDF Download

Compressibility Chart - an overview
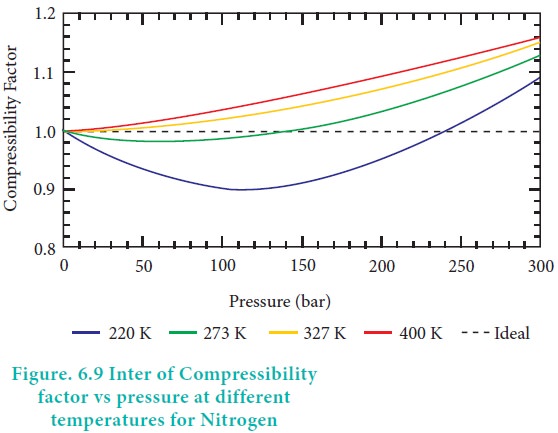
Compressibility factor Z - Gaseous State
What is the effect of pressure on real gas? - Quora

Gas compressibility factor Z: Ideal gas vs Real gas
11.3: Critical Phenomena - Chemistry LibreTexts

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

As the pressure approaching zero i.e., very low pressure, the curves plotted between compressibility factor Z and P n mole of gases have the following characteristics.I. The intercept on the y-axis leads