42g of N₂ react with excess of O₂ to produce NO. Amount of NO

Share your videos with friends, family, and the world
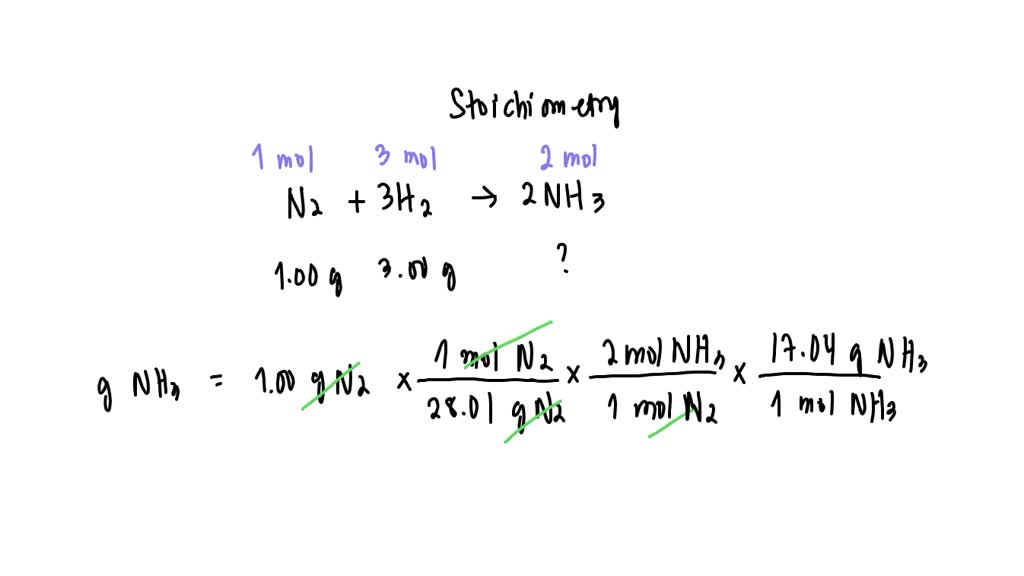
SOLVED: If 1.00 g of nitrogen gas react with 3.00 g of hydrogen gas to produce ammonia gas, what mass of ammonia will form

stoy-key-ahm-e-tree) - ppt download

Chemistry in Daily Life Homework Help, Questions with Solutions - Kunduz
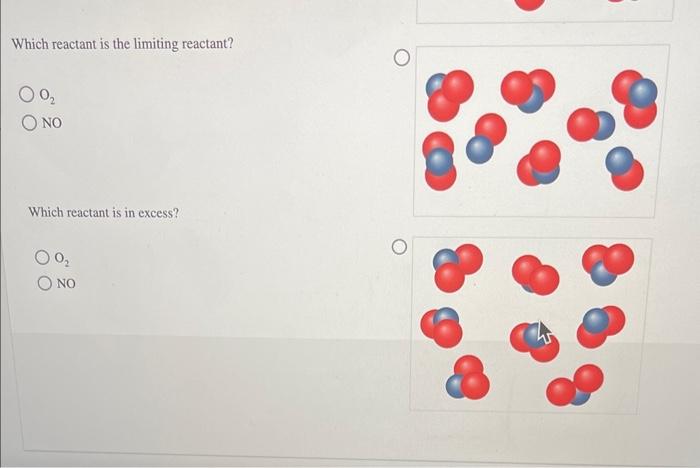
Solved reacts with NO to form NO2 according to the ollowing

Answered: Gaseous ammonia chemically reacts with…
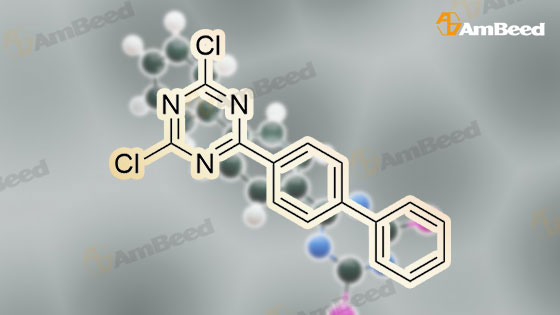
10202-45-6, 2-(4-Biphenylyl)-4,6-dichloro-1,3,5-triazine

Solved If 10.0 moles of O2 are reacted with excess NO in the
16433-96-8, 1-Ethynyl-2-nitrobenzene
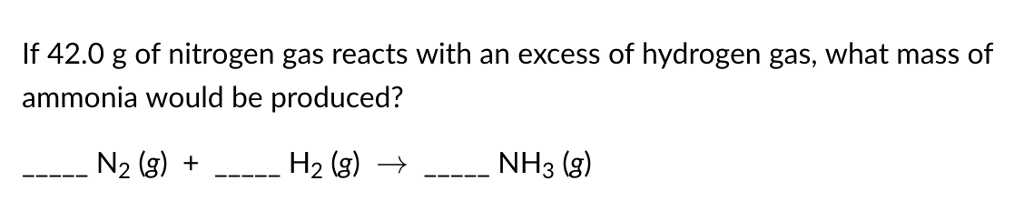
Solved If 42.0 g of nitrogen gas reacts with an excess of

42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g
How to calculate the maximum mass of ammonia, NH3, that could be made from 42 tonnes of nitrogen and excess hydrogen - Quora







