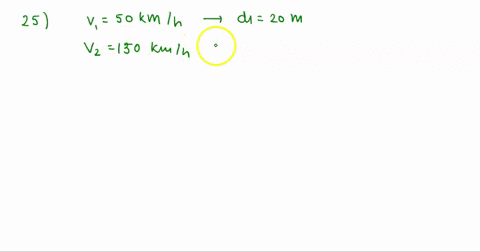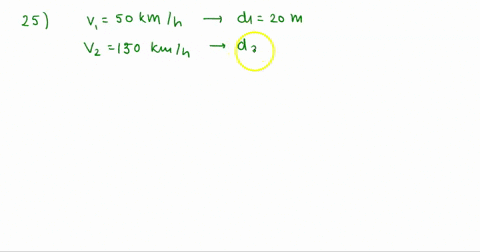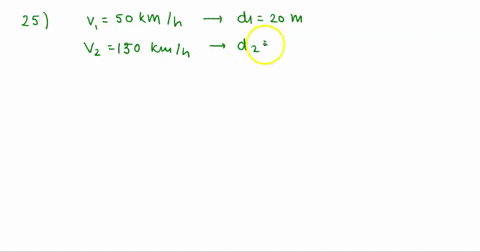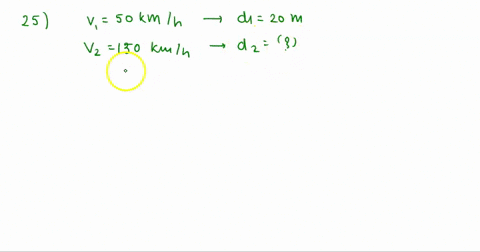
At Critical Temperature,pressure and volume . The compressibility
SOLVED: at critical temperature, pressure and volume the compressibility factor z is?

At critical temperature, pressure and volume. The compressibility factor (Z) is 2
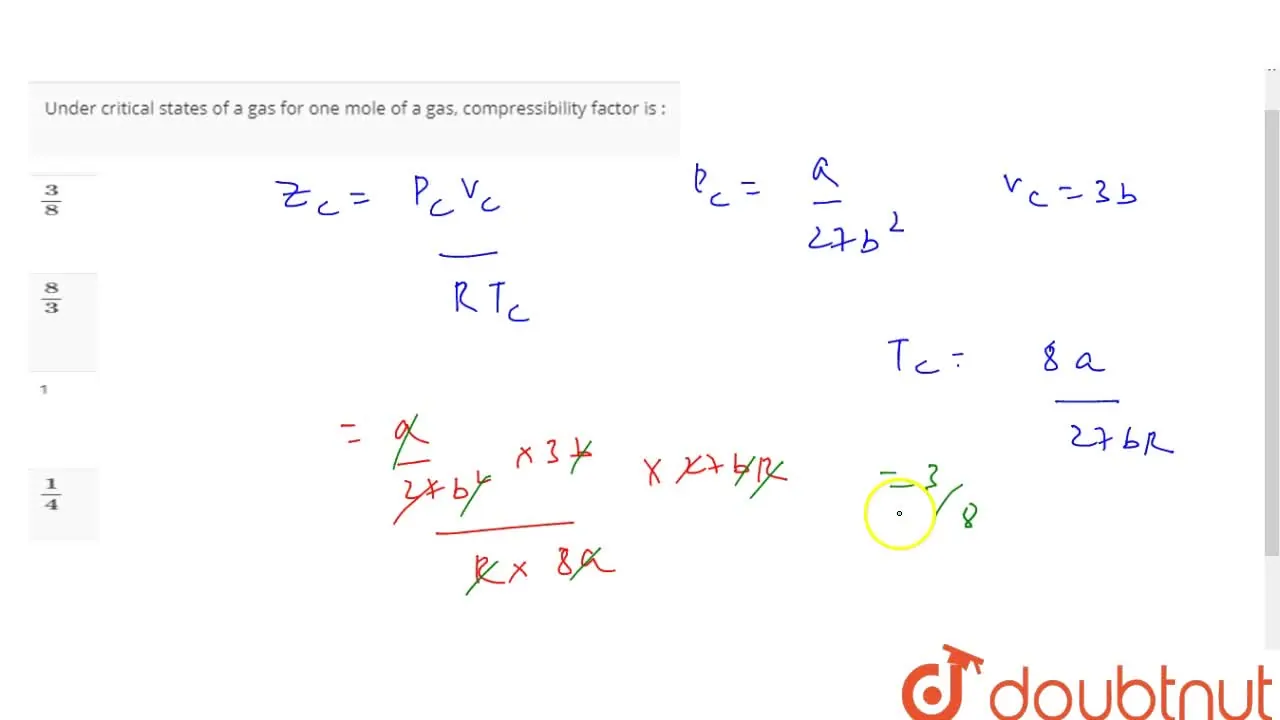
Under critical states of a gas for one mole of a gas, compressibility

ANSWERED] At critical temperature pressure and volume The - Kunduz

At critical temperature, pressure and volume. The compressibility factor (Z) is 2

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02

States of Matter study material with practice question 2023 - Chapter Contents Intermolecular - Studocu

A real gas has critical temperature and critical pressure as 40^(@)C a

Filo Student Questions For CBSE , Class 11 , Chemistry , Gase

States of Matter, PDF, Gases

At what temperature will be total kinetic energy (KE) of 0.30 mole of He ..

Critical temperature of a gas is. Boyle's temperature.

At critical temperature, pressure and volume. the compressibility factor z is











