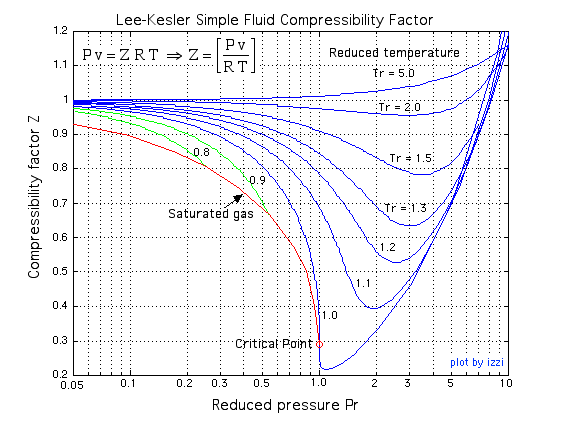
117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum

Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum
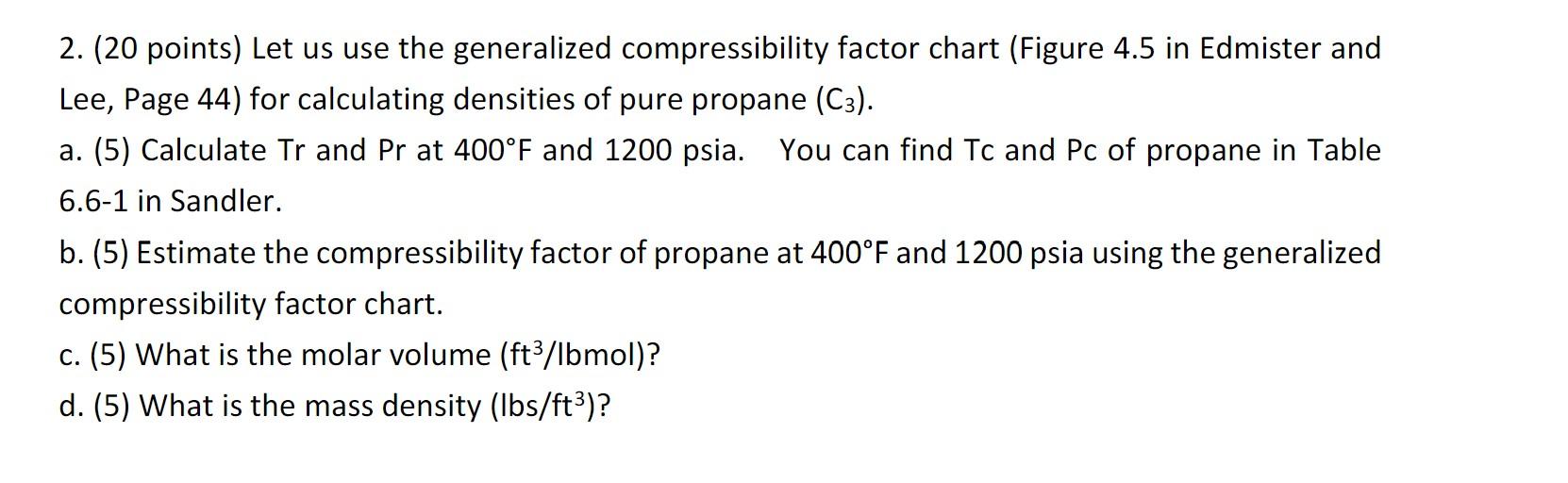
Solved Let us use the generalized compressibility factor

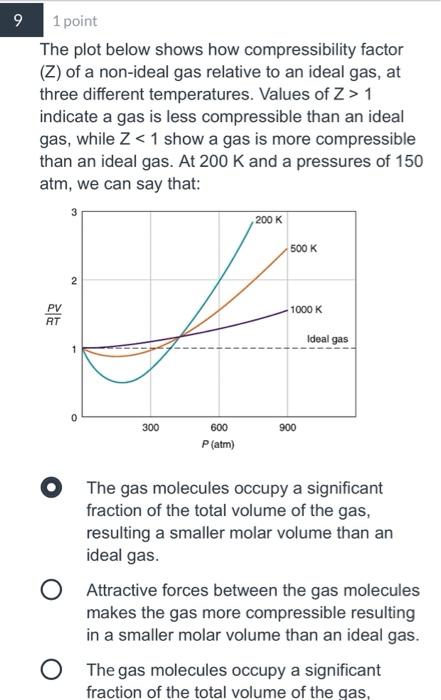
Solved The plot below shows how compressibility factor (Z)

Chemosensors, Free Full-Text

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 2 - Sarthaks eConnect
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Solved Data: Ideal gas constant R 8.314 J mol-1 K-1 1(a)
Solved 3 1 point Two gases, methane (CH4) and X, are

Chemosensors, Free Full-Text

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z=(1-displaystylefrac{a}{V _{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})