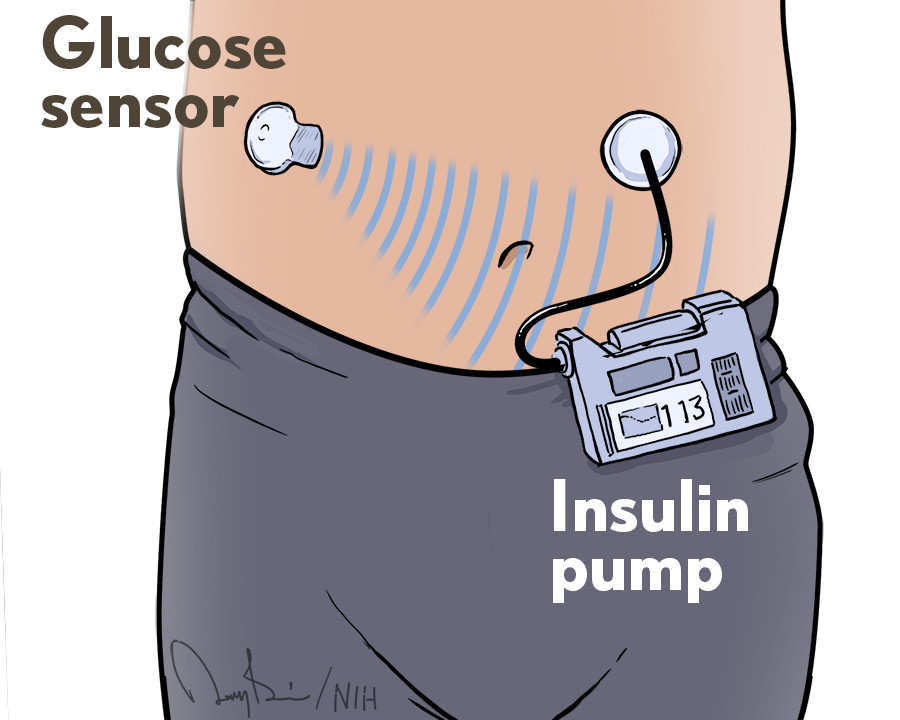
Medtronic recalls some insulin pumps that could lead to dangerous incorrect dosing
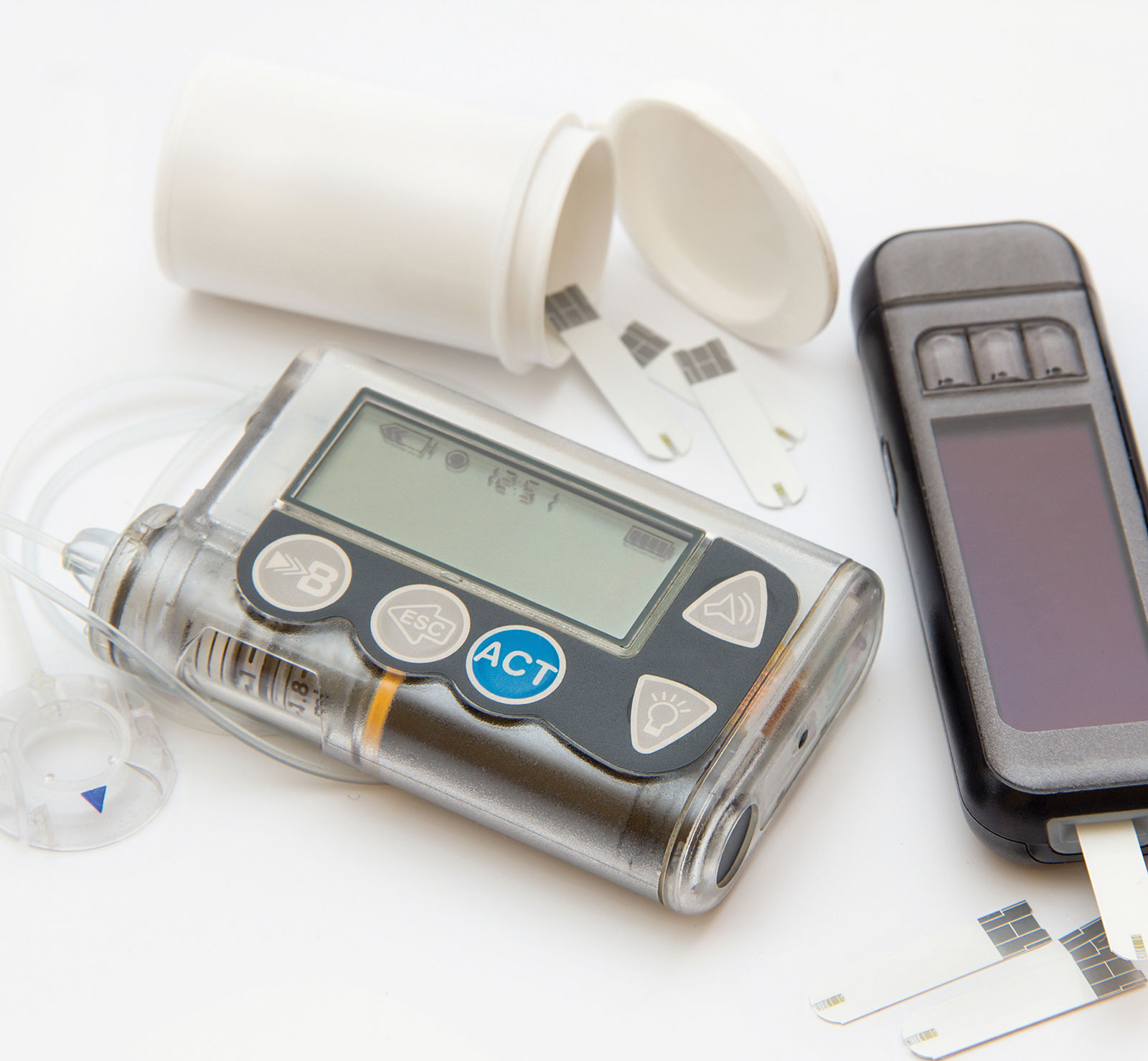
Medtronic is recalling MiniMed 600 Series Insulin Pumps. The FDA calls recall Class I the most serious type of recall, which can lead to injury or death.
This is additional taxonomy that helps us with analytics

Medtronic Insulin Pump Recall - The Sentinel Group
Understanding the Insulin Pump Recall
Medtronic recalls some insulin pumps that could lead to dangerous incorrect dosing

Medtronic recalls insulin pumps that could lead to fatal incorrect dosing

Medtronic recalls certain MiniMed insulin pumps tied to 1 death
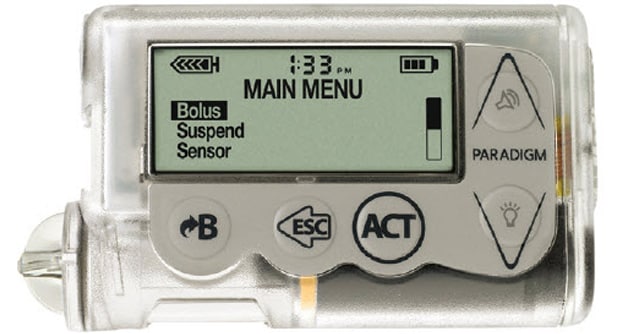
Explaining active insulin
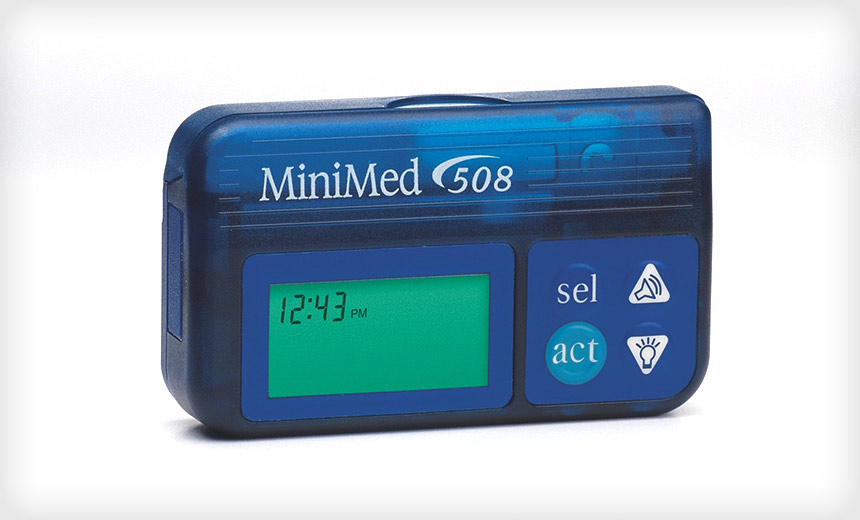
Medtronic Insulin Pump Devices Recalled Due to Serious Risks

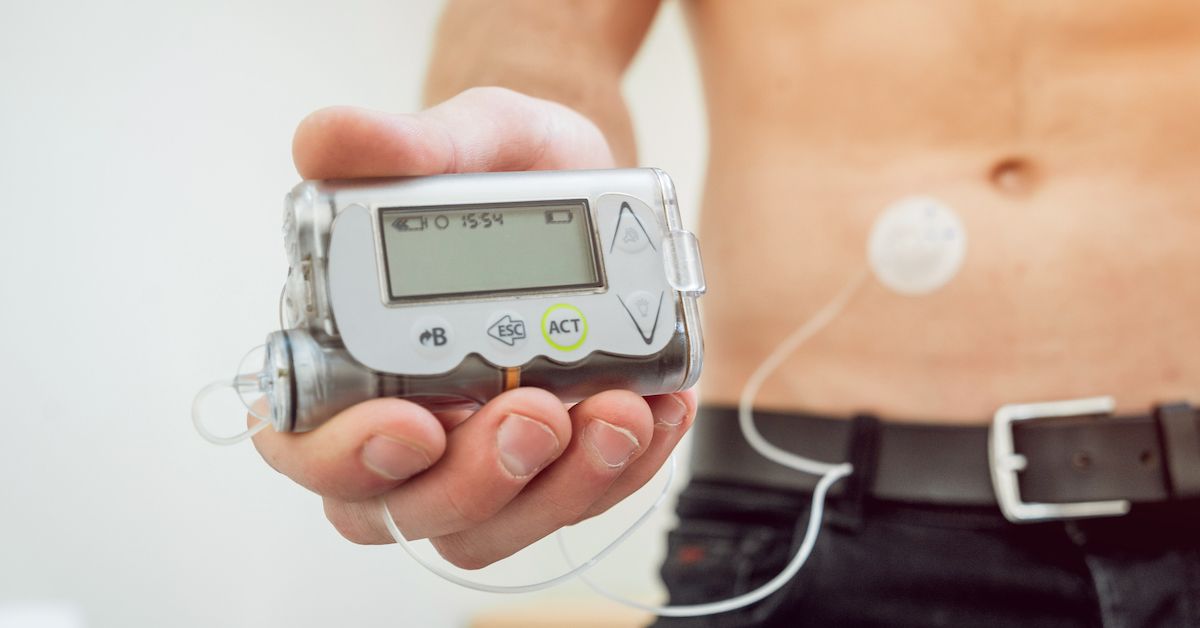
Insulin pumps, widely used, also have the most problems in FDA's database

Understanding the Insulin Pump Recall

Different Reviews: Medtronic 670G

FDA recalls insulin pumps tied to over 2,000 injuries and 1 death

Medtronic Insulin Pumps Recalled After Dangerous Flaw Leaves One Dead - Legal Reader

Medtronic Recalls MiniMed Insulin Pumps for Incorrect Dosing Risk – NBC Connecticut

Medtronic MiniMed Insulin Pump Lawsuit March 2024 - Select Justice

Medtronic recalls some insulin pumps that could lead to dangerous incorrect dosing