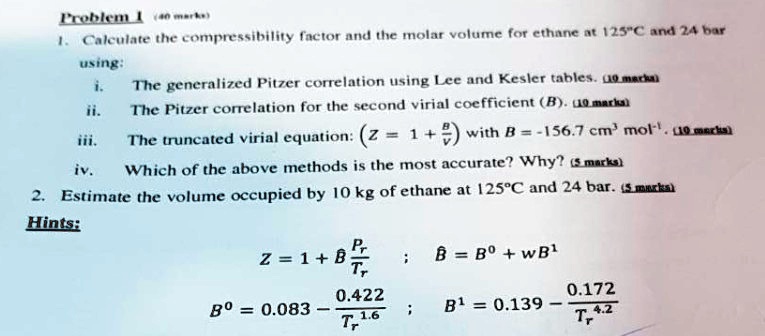
At low pressure, the van der waal's equation is written as (P+ a/V
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to
Van der Waals Equation: Derivation, Correction Factor, Significance

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0

Van der Waals equation - Wikipedia

Van Der Waals Equation - an overview

Van Der Waals Equation - an overview
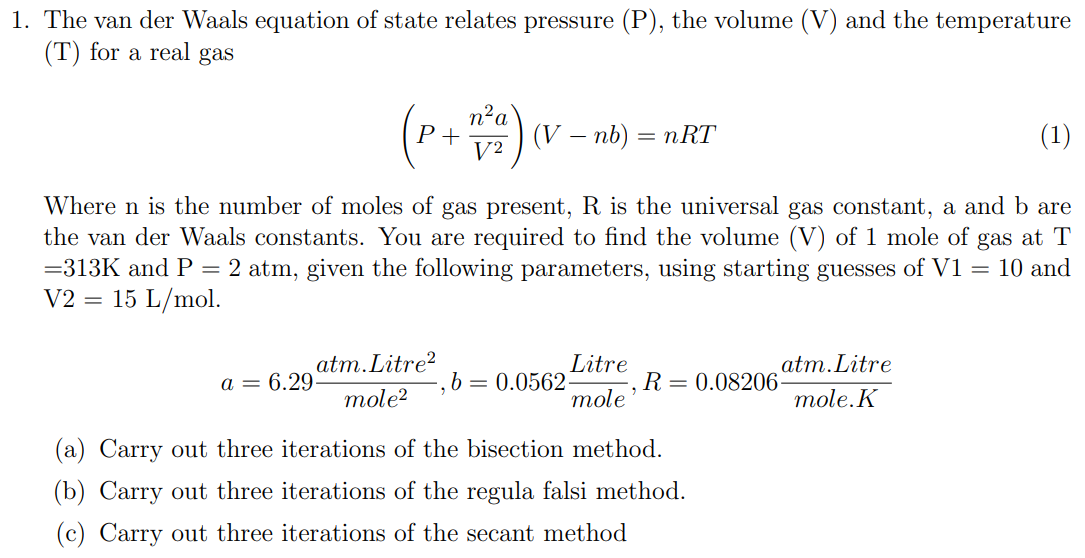
Solved The van der Waals equation of state relates pressure
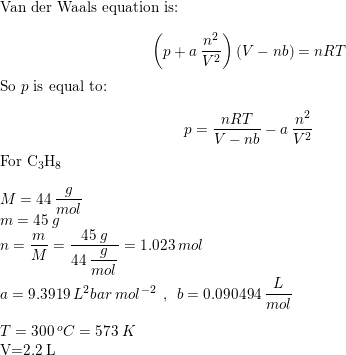
Use the van der Waals equation to calculate the pressure in
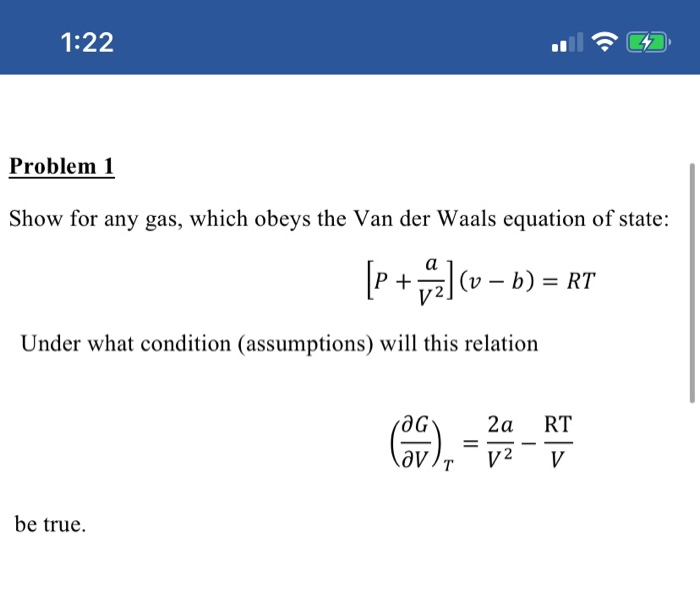
Solved show for any gas, which obeys the Van der Waals
Van der Waals Equation, Virial Expansion

The Van der Waals equation a real gas is : (P + 2) (V – b) = RT

3) Zone refining Cupellation Compressibility factor of carbon dioxide gas 0°C under low pressure is equal to 1148. (2) Pb (1) 1 (2) RT a (3) 1 RTV As 1-RT 49. Which of the following radicals is least stable?

At low pressure, the van der Waal's equation become
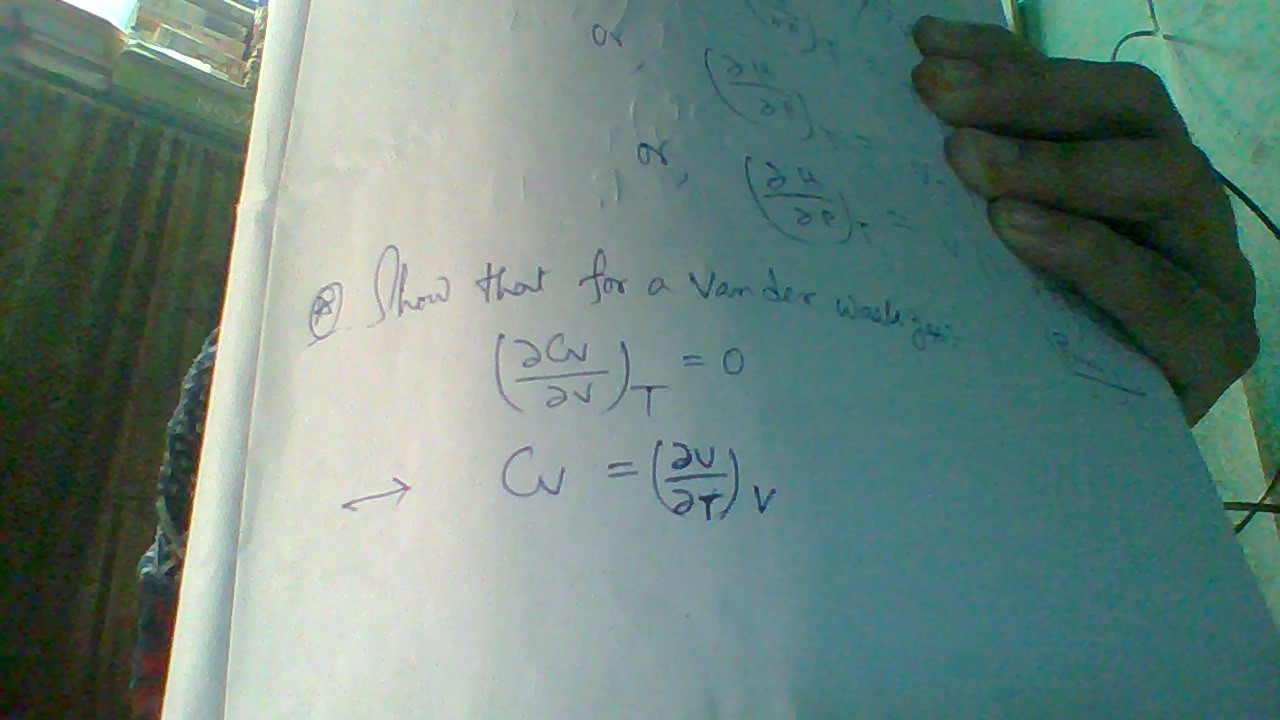
Show that for a van der Waals gas, ((delC_V)/(delV))_T = 0, where

Vander-Waals Equation of State - GATE ME '15 S1
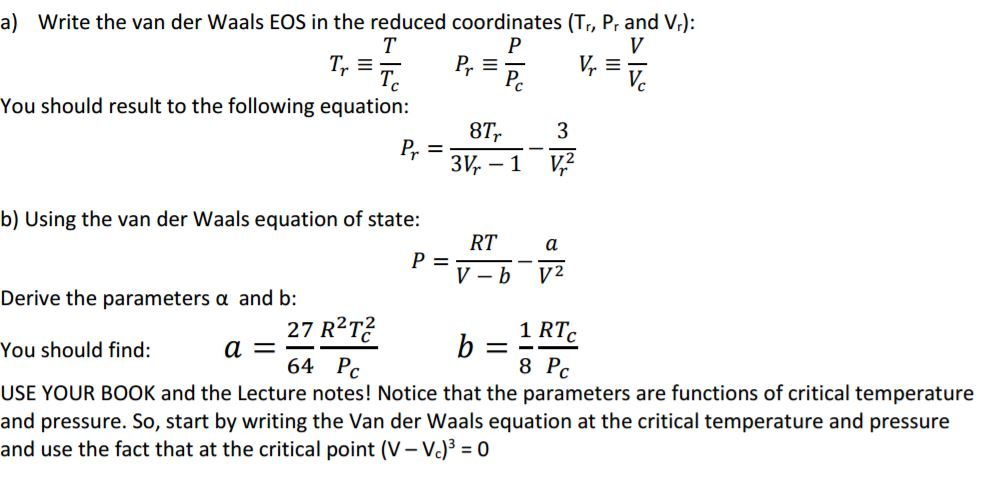
Solved Write the van der Waals EOS in the reduced