For each of the negative ions listed in column 1, use the periodic table to find in column 2 the total number of electrons the ion contains. A given answer may be


SOLVED: For each of the positive ions listed in column 1 use the periodic table to find in column 2 the total number of electrons that ion contains. The same answer may
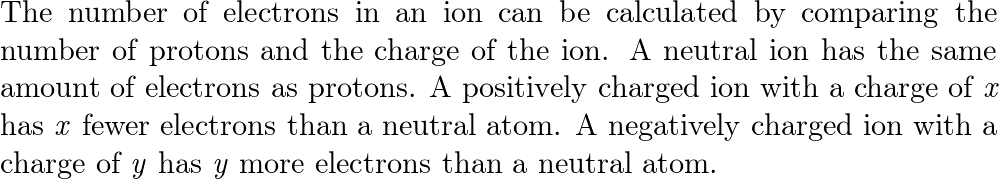
Fill in the blanks to complete the table., Symbol, Ion Comm

Introductory Chemistry 7th Edition

Chem Unit 3 Ions Answers - Standards: 3.1.10 B Describe concepts of models as a way to predict and understand science and technology. 3.4.10 A Explain

Chapter 5, Nomenclature Video Solutions, Introductory Chemistry
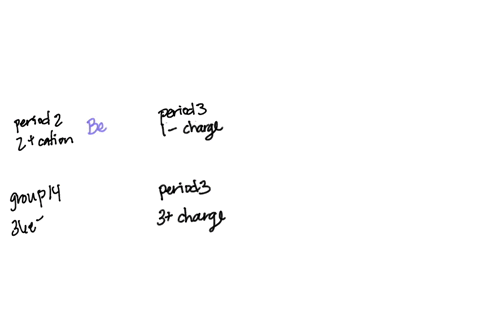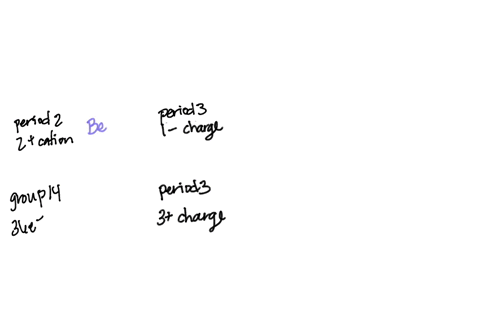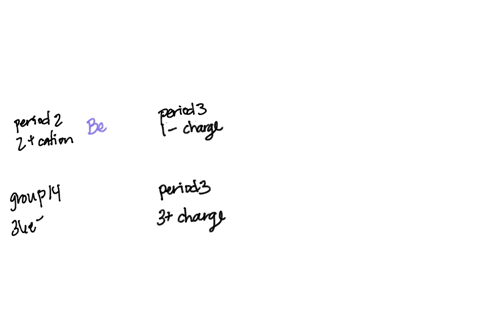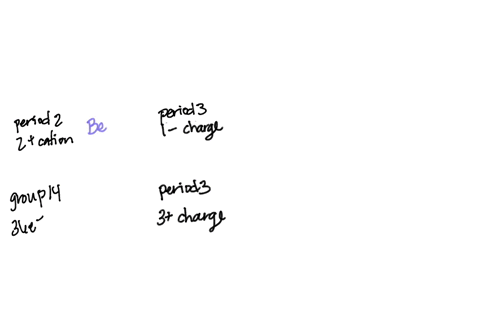
SOLVED: Question 24 1 pts An atom of an element forms stable ion by easily losing electrons. The ion has charge 2 and is in Group 2 2 and is in Group
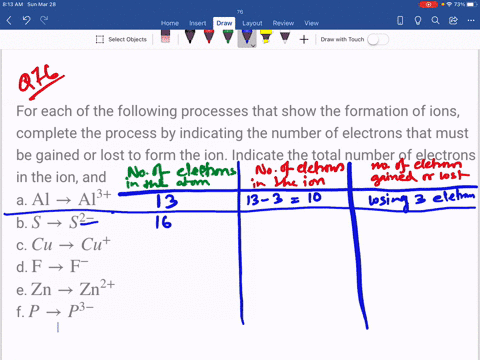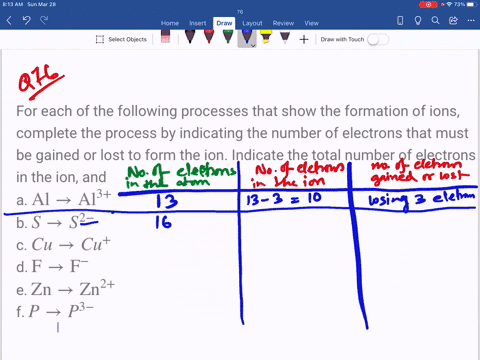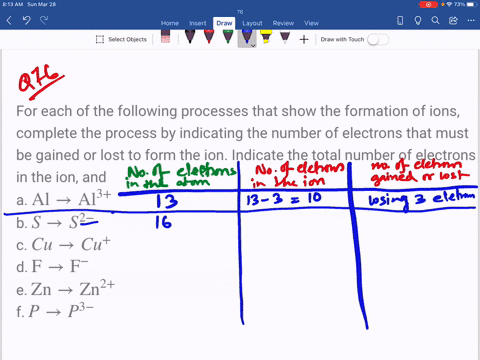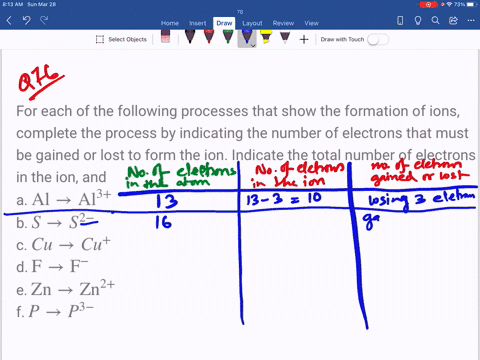
⏩SOLVED:For each of the negative ions listed in column 1, use the…
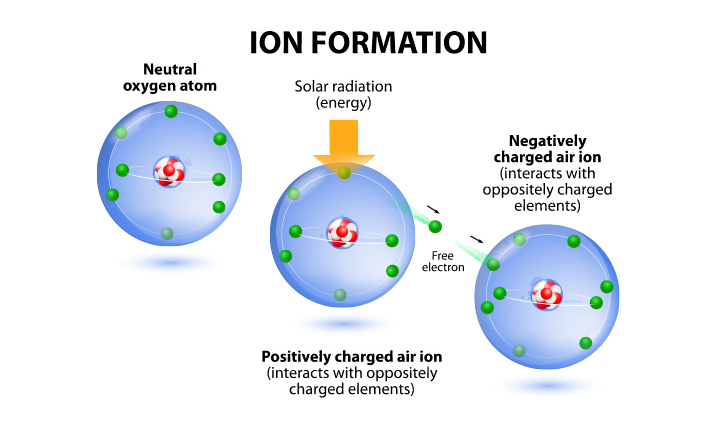
Atomic Structure d. Atomic Structure d Atomic Structure d Electron (negative) Neutron (neutral) Proton (positive) d nucleus. - ppt download

Chapter 5, Nomenclature Video Solutions, Introductory Chemistry
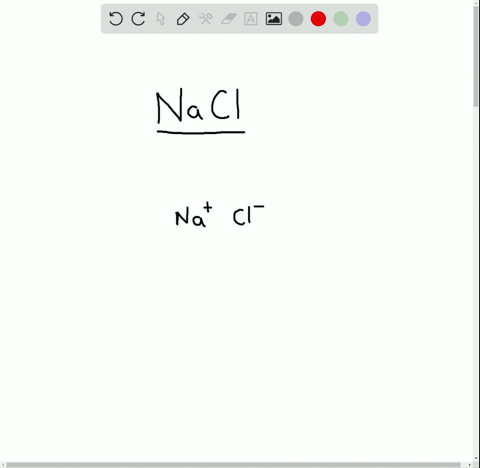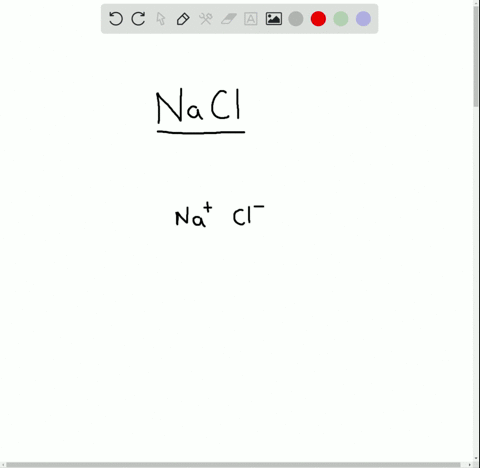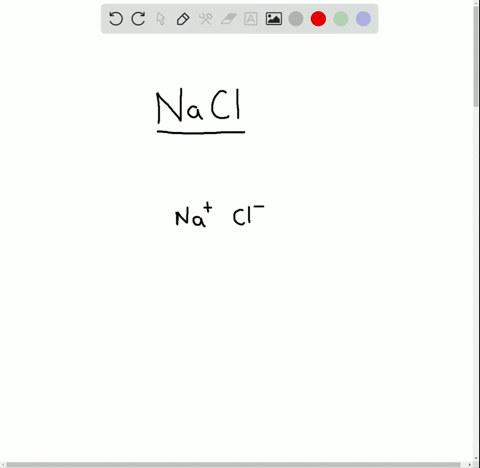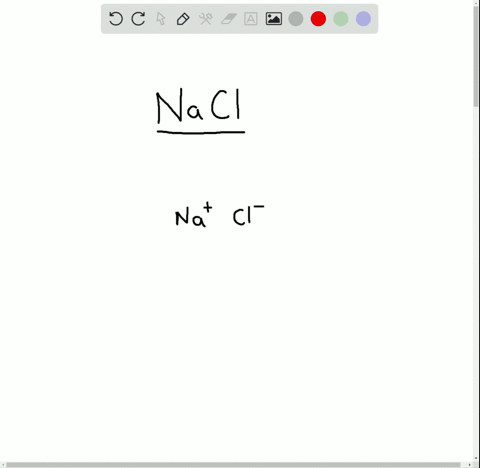
Chapter 5, Nomenclature Video Solutions, Introductory Chemistry
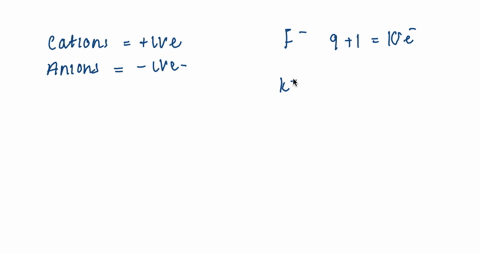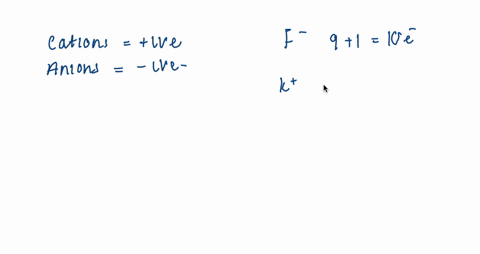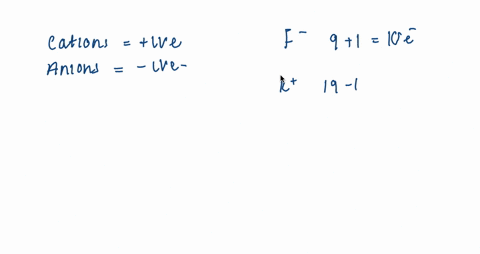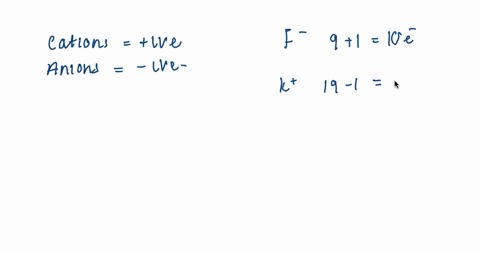
⏩SOLVED:For each of the negative ions listed in column 1, use the…

Fill in the blanks to complete the table., Symbol, Ion Comm

SOLVED: An ion is formed when an atom gains or loses an electron or electrons. Ions have a charge. If an atom has seven electrons in the outer shell, it will tend
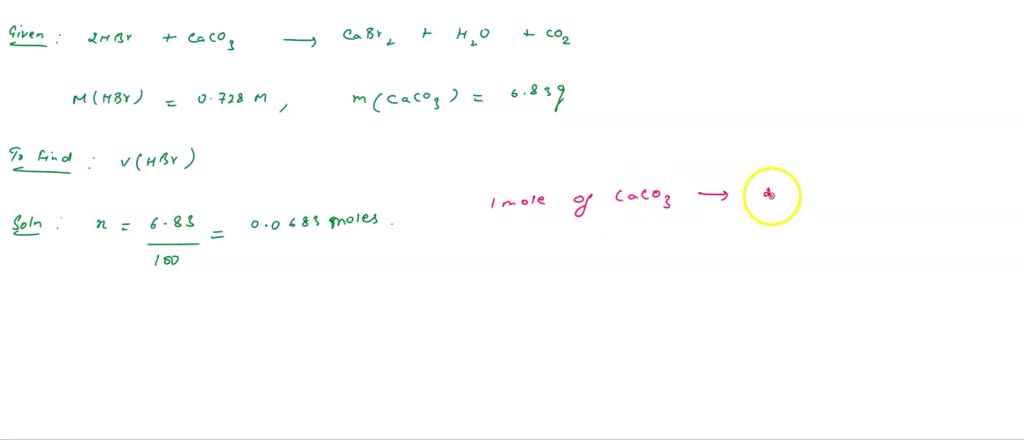
SOLVED: How many mL of 0.728 M HBr are needed to dissolve 6.83 g of CaCO3?

SOLVED: Group 64 Enter signed integer: For example, +2 or -1 Constants Periodic Table: Locate each of the following groups on the periodic table and list the charge of the ions they