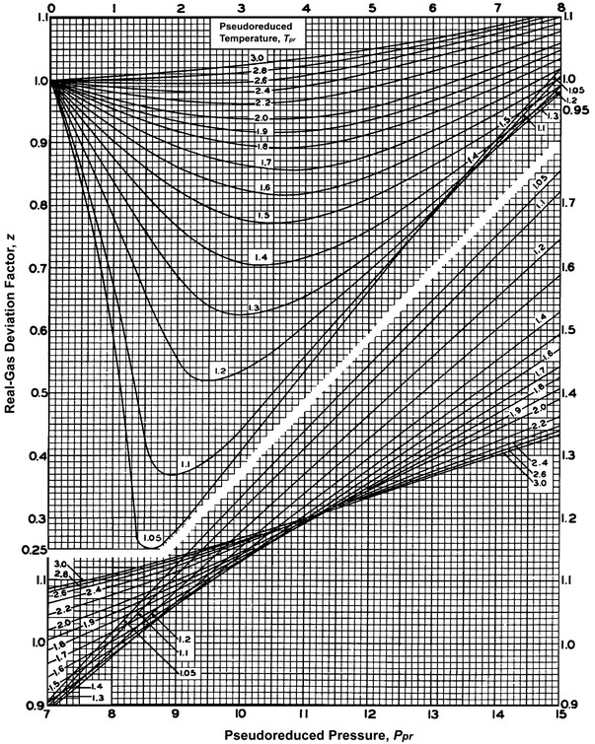
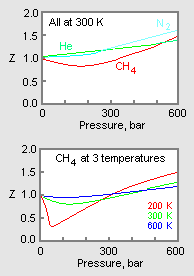
1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

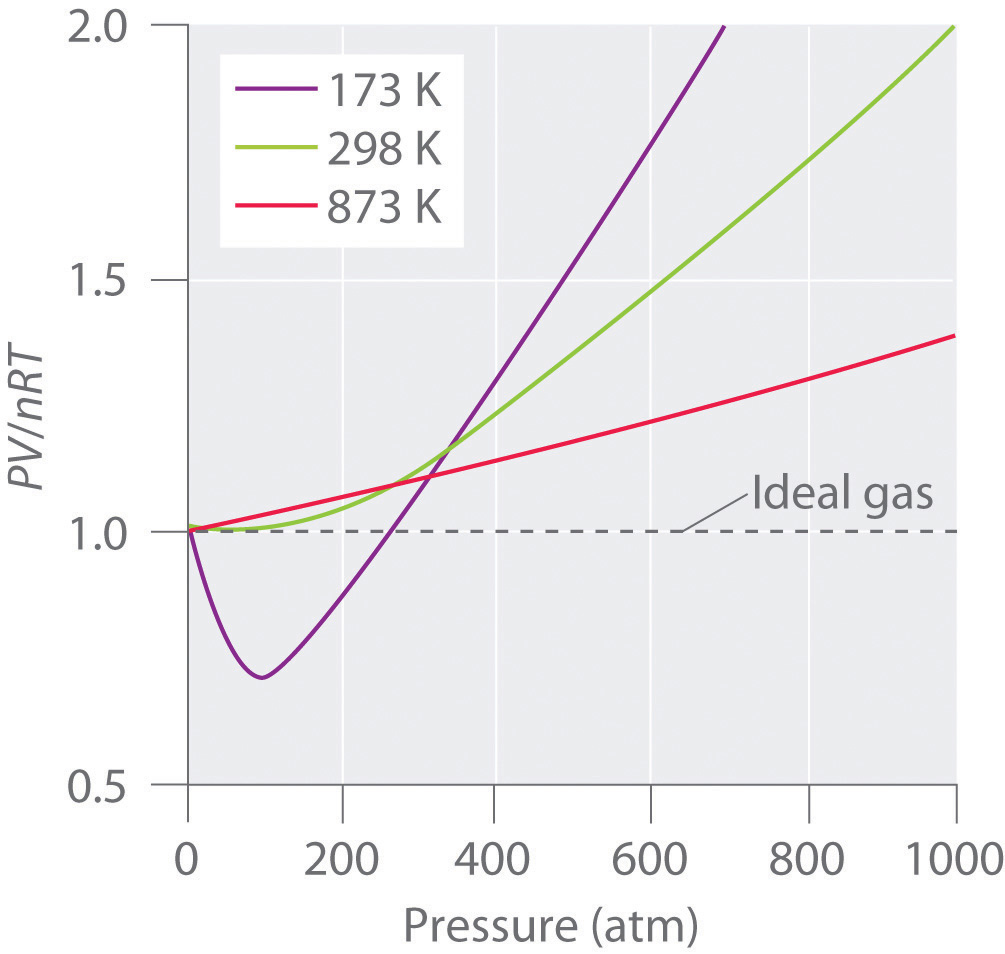
Chapter 11.1: Real Gases - Chemistry LibreTexts

Chapter 11.1: Real Gases - Chemistry LibreTexts
How do Van der Waals constants a and b depend on temperature, pressure and volume? - Quora

Van der Waals Equation Practice Problems

The Boyle Temperature in a two-term virial equation of state
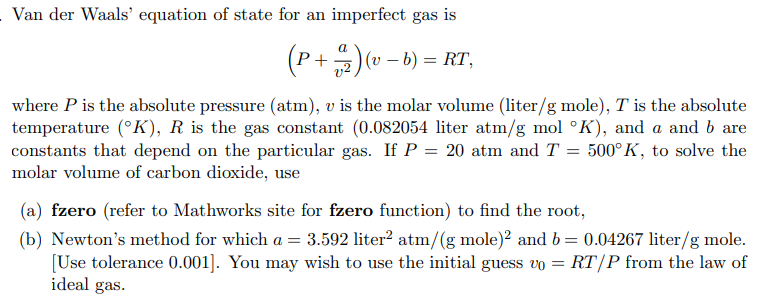
Solved Van der Waals' equation of state for an imperfect gas
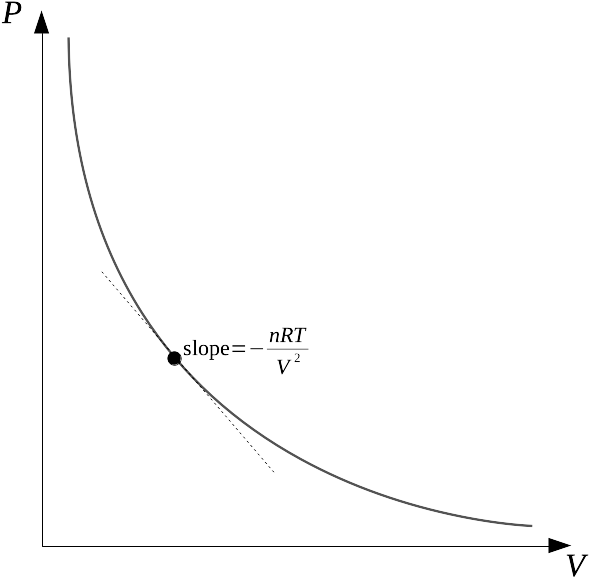
1.8: The ideal gas law, functions and derivatives - Chemistry LibreTexts
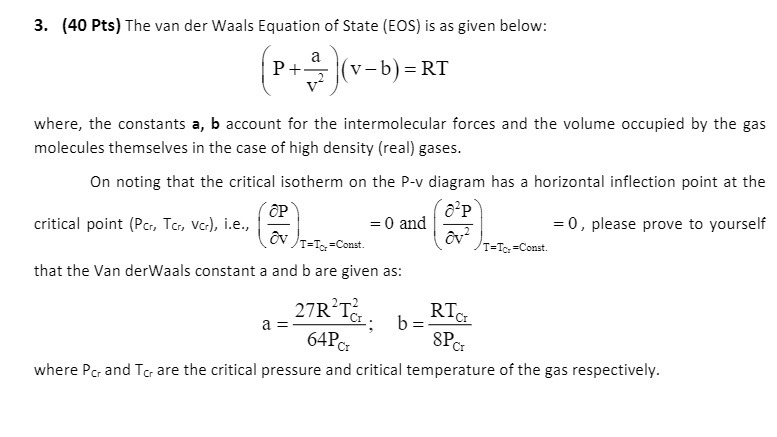
SOLVED: Please prove to yourself that the Van der Waals constants a and b are given as: 3. (40 Pts) The van der Waals Equation of State (EOS) is as given below: (
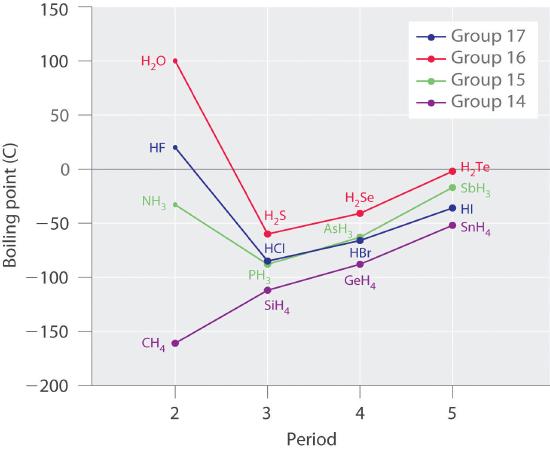
11.2: Intermolecular forces - Chemistry LibreTexts
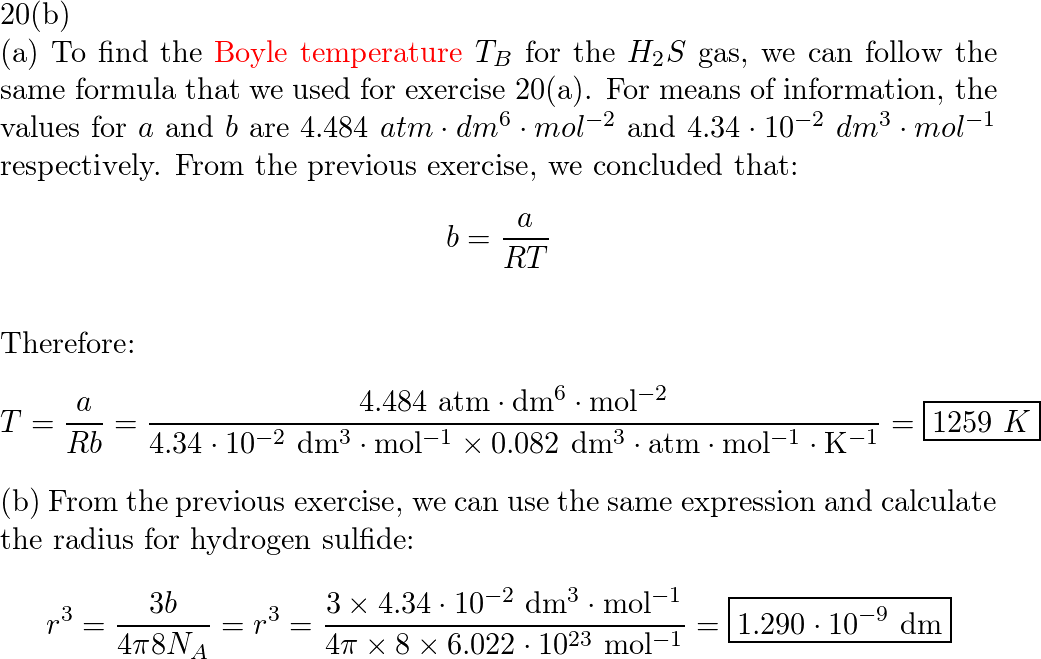
a) Use the van der Waals parameters for chlorine of the Res

DUJS 20F Print Journal by dartmouthjournalofscience - Issuu