Low coverage whole genome sequencing enables accurate assessment
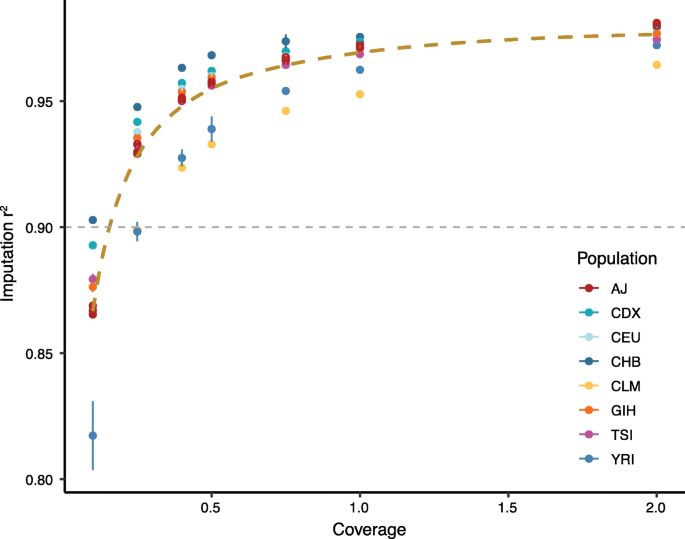
Background Inherited susceptibility to common, complex diseases may be caused by rare, pathogenic variants (“monogenic”) or by the cumulative effect of numerous common variants (“polygenic”). Comprehensive genome interpretation should enable assessment for both monogenic and polygenic components of inherited risk. The traditional approach requires two distinct genetic testing technologies—high coverage sequencing of known genes to detect monogenic variants and a genome-wide genotyping array followed by imputation to calculate genome-wide polygenic scores (GPSs). We assessed the feasibility and accuracy of using low coverage whole genome sequencing (lcWGS) as an alternative to genotyping arrays to calculate GPSs. Methods First, we performed downsampling and imputation of WGS data from ten individuals to assess concordance with known genotypes. Second, we assessed the correlation between GPSs for 3 common diseases—coronary artery disease (CAD), breast cancer (BC), and atrial fibrillation (AF)—calculated using lcWGS and genotyping array in 184 samples. Third, we assessed concordance of lcWGS-based genotype calls and GPS calculation in 120 individuals with known genotypes, selected to reflect diverse ancestral backgrounds. Fourth, we assessed the relationship between GPSs calculated using lcWGS and disease phenotypes in a cohort of 11,502 individuals of European ancestry. Results We found imputation accuracy r2 values of greater than 0.90 for all ten samples—including those of African and Ashkenazi Jewish ancestry—with lcWGS data at 0.5×. GPSs calculated using lcWGS and genotyping array followed by imputation in 184 individuals were highly correlated for each of the 3 common diseases (r2 = 0.93–0.97) with similar score distributions. Using lcWGS data from 120 individuals of diverse ancestral backgrounds, we found similar results with respect to imputation accuracy and GPS correlations. Finally, we calculated GPSs for CAD, BC, and AF using lcWGS in 11,502 individuals of European ancestry, confirming odds ratios per standard deviation increment ranging 1.28 to 1.59, consistent with previous studies. Conclusions lcWGS is an alternative technology to genotyping arrays for common genetic variant assessment and GPS calculation. lcWGS provides comparable imputation accuracy while also overcoming the ascertainment bias inherent to variant selection in genotyping array design.

Ultra-low-coverage genome-wide association study—insights into gestational age using 17,844 embryo samples with preimplantation genetic testing, Genome Medicine
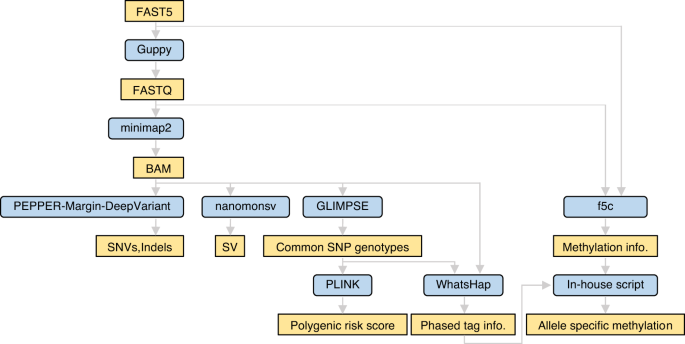
Assessing the efficacy of target adaptive sampling long-read sequencing through hereditary cancer patient genomes
Copy Number Variation (CNV) Analysis: A Guide for Clinical Researchers
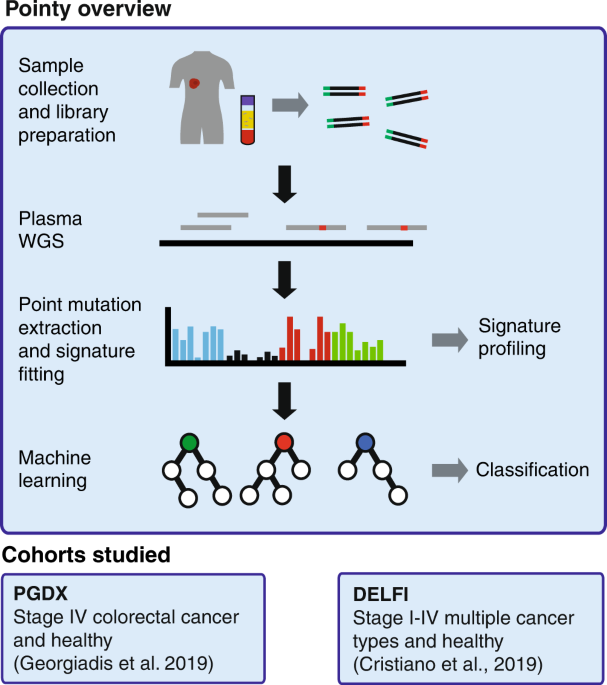
Genome-wide mutational signatures in low-coverage whole genome sequencing of cell-free DNA

Ultra-low-coverage genome-wide association study—insights into gestational age using 17,844 embryo samples with preimplantation genetic testing, Genome Medicine

NGS-based accurate and efficient detection of circulating cell-free mitochondrial DNA in cancer patients - ScienceDirect

Evaluating genotype imputation pipeline for ultra-low coverage ancient genomes

Identification of RP1 as the genetic cause of retinitis pigmentosa in a multi-generational pedigree using Extremely Low-Coverage Whole Genome Sequencing (XLC-WGS) - ScienceDirect

Relative matching using low coverage sequencing
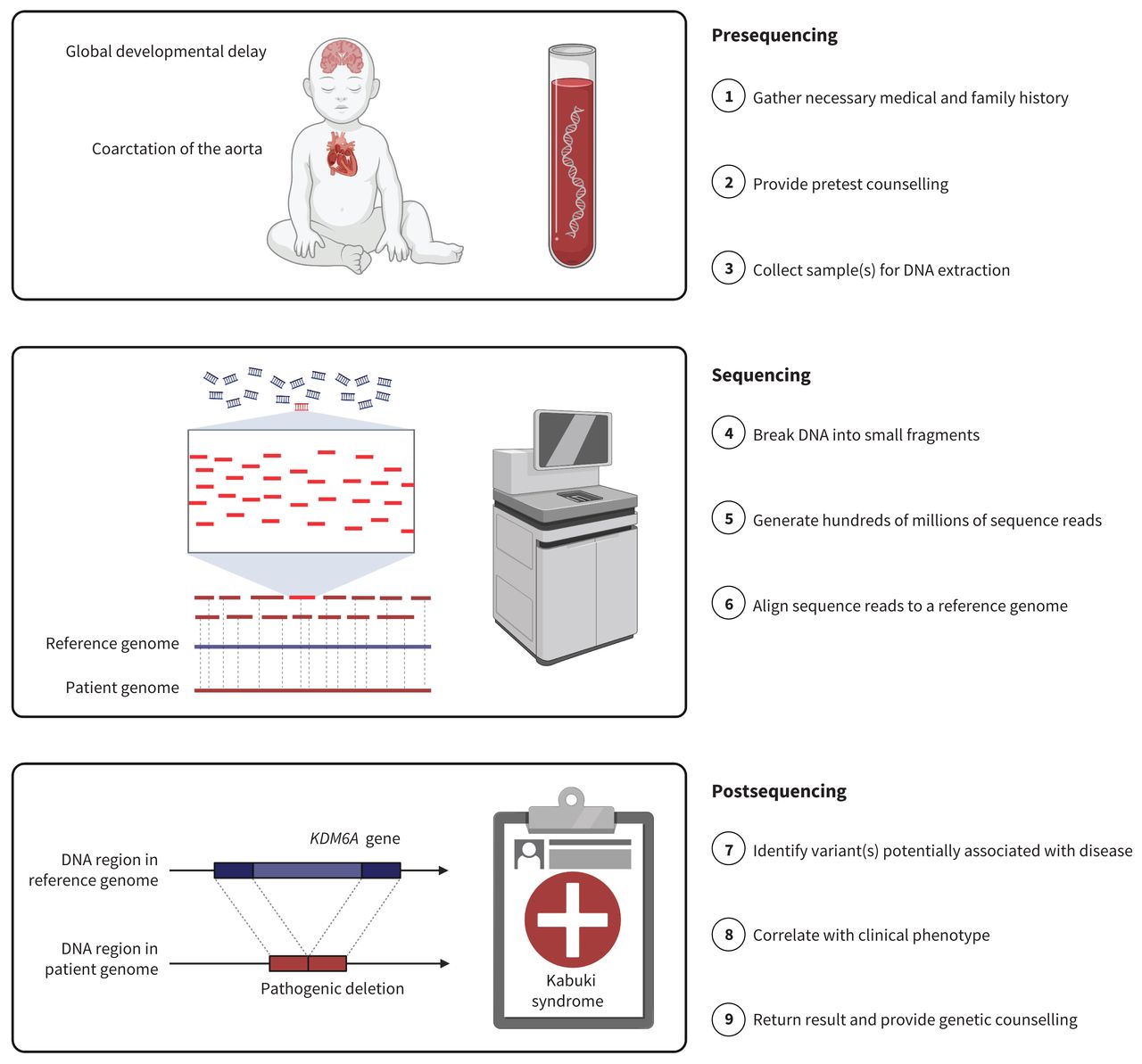
Genome sequencing as a diagnostic test